Although a number of entities and organizations—including governments, professional societies, hospitals, and consumer advocacy organizations—have developed consumer reporting tools to elicit information that might inform patient awareness and improvement initiatives,39, 41, 43, 44 no well-established model exists for eliciting consumer-identified patient safety events on a national scale, as envisioned by AHRQ. Therefore, with support from AHRQ and knowledge gleaned from prior efforts, the project team undertook the design and development of a new prototype for a consumer hotline for reporting patient safety events. The first phase of the effort, design and development, was completed between September 2011 and September 2013.
From the beginning of the design phase, the research team intended for the hotline to enable patients and caregivers to voluntarily report their safety concerns through an event reporting form that could be completed online or by phone. The hotline was designed to focus on care across a continuum of settings (hospitals, physician practices, etc.) and to include care provided to both adults and children. The hotline had four key building blocks:
- A patient event reporting form.
- A Web-based data collection platform.
- Protocols for data collection, processing, and sharing.
- Legal and regulatory protections for the data.
During the development process, the research team had to devise strategies to address many challenges that arose. These included the complexities of the legal context for collecting patient and provider information on safety concerns (including issues of consent and confidentiality), the need to develop language and format acceptable to consumers, and the need to test various report solicitation approaches. We developed procedures for classifying events and for identifying reports about the same events within the health care delivery organizations and devised ways for this information to be protected within a PSO structure. We also developed a marketing campaign and community-specific outreach materials and previewed the Web site interface with patients, families, and caregivers.
This chapter begins with a discussion of design considerations that influenced our work. We then discuss the methods used to develop each of the four key building blocks.
Design Considerations
Translating the observations of patients and caregivers into usable data for safety improvement required maximizing the utility of the voluntarily reported observations while also protecting sensitive information that must be kept confidential under Federal and State laws. A few of the most important design considerations are noted here.
Acceptability of a Hotline to Patients and Caregivers
To be effective, a patient safety hotline must be acceptable to patients and caregivers. In particular, the process used to elicit a report must be easy to understand, and it must be complete. It must protect privacy by ensuring that the person who makes the report (patient, family member, or other caregiver) controls the information that is shared. It should allow for anonymous reporting or offer confidentiality, and it should also offer an option for the patient or caregiver to provide identifying information to enable followup with both the patient and providers who may have been involved in the reported events.
Awareness of Obligations Created by Patient Reporting
The architecture of a patient reporting system must take into account the fact that patient-reported information about patient safety-related health care experiences is not subject to the same peer-review protections as information that health professionals submit to hospital-based adverse event reporting systems.17 To the extent that patient reports represent grievances under CMS Conditions of Participation (Medicare), health care delivery organizations are obliged to respond in a timely and formal manner.e While health care organization analyses of patient safety reports are protected under many State peer-review protection laws and by the Federal PSO umbrella, the initial reports themselves may represent a potential liability risk that health delivery organizations will need to take into account.f
Prompted Versus Passive Outreach Strategies
The method used to solicit reports may affect the number and type of reports received. The research literature shows that a prompted reporting method (i.e., actively soliciting reports from subjects in person or by phone outreach) can result in higher patient participation.1, 29, 31 In contrast, passive solicitation strategies, which involve simply making a Web site or phone number available, typically achieve substantially lower reporting rates. Because the reports are likely to come from highly motivated patients and caregivers, the sample may not be representative of the range of patient safety issues occurring in practice. Low response rates also suggest underreporting of events, and when the number is very small, it is difficult to track trends reliably over time. Approaches that incorporate aggressive public education and outreach may achieve higher reporting rates, although this strategy has not been fully evaluated. To date, no study has specifically compared prompted and passive solicitation modalities. In addition, national and locally based reporting systems may perform differently, as patients and families may be more motivated to report to local systems, where they have greater affiliation and perceive greater potential to benefit personally from improvements.
Balancing Multiple Objectives
A patient safety reporting system created to support health system-based improvements has a different aim than one intended primarily to make information freely available to the public. A system designed to provide information to the public might have little screening or editing and would seek to make reports quickly and efficiently available to those potentially seeking care. Patient and caregiver ratings could be aggregated and displayed on Web sites such as Yelp or in publications such as Consumer Reports. In contrast, a system designed to support patient safety improvements within a health delivery operation would seek to combine patient reports with other sources of information and would prioritize peer review, regulatory compliance, and quality improvement over public reporting and public accountability.
Creating the Event Reporting Form
We used multiple methods to develop the new event reporting form for collecting patient safety observations from patients and caregivers. We modeled the form, in part, on a survey previously developed by three of the research team members (Weissman, Schneider, and Weingart) for a study of patient-reported adverse events in Massachusetts.1 We first conducted an environmental scan (i.e., a review of existing instruments) and reviewed development guidelines for patient surveys. Then, after we developed the initial form, we elicited feedback from two focus groups. We used a series of cognitive interviews to refine the questions and revised the draft form in response to the interviews and feedback from technical experts, patient advocates, and the public. We provide more information on each of these methods in the following sections.
Environmental Scan
The research team conducted an environmental scan of existing patient safety reporting instruments to identify the state of the art in patient safety reporting. We also reviewed the minimum dimensions for adverse event reporting recommended by IOM and the AHRQ Common Formats.g
We examined instruments for collecting adverse events, errors, near misses, and unsafe conditions in the United States and other countries and identified 27 consumer-enabled reporting systems and 14 survey tools with questions about health care safety. We focused on event type (e.g., medication or device concerns, clinician or facility concerns), reporting mode (e.g., online, phone), key terms used (e.g., mistake, problem, near miss), and specific health care patient safety survey items.
We found none of the existing data collection tools to be completely satisfactory for the purpose at hand. Our prior work suggested the importance of distinguishing injury (negative effects) from errors, which we corroborated in subsequent focus groups. We used the literature review and the environmental scan to develop a catalog of potential questions, from which we created a limited set. Our primary concerns were the clarity of the questions, their face validity, and the frequency of reported response categories. We selected response items that we believed to be most commonly encountered and reported. Because of space constraints, we could list only a limited number of response elements. Each category included an open-ended response; our intent was to update the form in future iterations, based on the number and type of responses received.
Patient and Caregiver Focus Groups
In December 2011, we conducted two focus groups with patients and patients’ family members. The first focus group took place in Boston and was attended by 12 English-speaking adults who had either experienced a safety event personally (n=7) or had a family member who had experienced a safety event (n=5) in the previous 12 months. The second focus group took place in Los Angeles and was attended by nine Spanish-speaking adults who had either experienced a patient safety event personally (n=5) or had a family member who had experienced a safety event (n=4) in the previous 12 months. Participants in both groups were diverse with regard to gender, race/ethnicity, and education.
In advance of the focus groups, we gave the participants a short reporting form (which could be completed in 5 to 10 minutes) that asked about patient safety concerns they had experienced. The responses provided us with an idea of the breadth of the participants’ experience and their specific concerns. The form also focused the participants’ attention on the matter at hand and essentially primed them for the discussion to follow. During the focus groups, a moderator used exploratory and confirmatory approaches to investigate terminology, concepts related to patient safety and relationships between the concepts, discovery of patient safety events, reporting preferences, awareness of existing reporting systems, and the draft reporting form.
Cognitive Interviews
To inform the choice of specific terminology to be used in the patient event reporting form, the order of topics, and the wording of individual items, we conducted seven cognitive interviews. Cognitive interviews are a form of pre-testing in which the investigator asks a sample of likely respondents how they understood the questions that were asked and then compares their responses to the intent of the questions, in order to identify any problems with formatting, comprehension, or acceptability. The cognitive interviews took place in February and March 2012. All interviews were conducted in English; four of the interviewees had experienced a patient safety event, and three were family members. Participants were diverse with regard to gender and education, as well as type of patient safety event. We tested interviewee understanding of specific terminology, the flow and redundancy of questions, and the interpretation of items on the event reporting form.
Design Changes in Response to Patient and Family Member Feedback
The research team made a number of changes in the design of the event reporting form in response to the input received. For example, focus group participants did not mention events with adverse or negative effects when they were asked about patient safety issues. They understood the concepts of “medical mistake” and “harm/injury” more clearly than they understood “patient safety.” We found that the term “patient safety concern” elicited comments on service complaints and communication issues. Both focus groups found the term “complication” ambiguous. Participants agreed that three categories of patient safety concerns were understandable and sufficient: medical mistakes, harm or injury, and unsafe conditions.
We therefore revised the initial reporting form to use the terms “mistake” and “harm” throughout, added specific questions about setting, and added a question about whether a patient was told about possible negative effects. The revised form was designed to ensure that a patient or caregiver would report only one concern or event at a time.
The focus group findings also confirmed the need to target the reporting form to the seventh-grade reading level, offer Spanish and English versions, offer multiple modes of reporting (i.e., Web and phone), follow up with those who report an event, and prioritize patient confidentiality within the system.
We also refined question content in response to the cognitive interviews. We focused the content and shortened the number of structured questions from 69 to 25. We also reordered the topics, rephrased and simplified wording, and limited response set lists to no more than five items. Open-ended questions with free-text narrative boxes were included at the beginning of the reporting form to allow patients and caregivers to tell what happened in their own language, and we limited the list of “contributing factors” to those that patients and families can observe directly and report on reliably.
External Review
In March 2012, the research team convened the first in-person meeting of the TEP. (The TEP members and their affiliations are listed in Appendix B.) The TEP members provided multi-stakeholder expertise on relevant dimensions of the project, including patient safety, reporting systems, patient perspectives, and survey methodology. The following topics were discussed during the initial in-person meeting: background and policy context, project goals and timeline, opportunities for stakeholders, instrument development, sections of the draft event reporting form, outstanding design issues, and criteria for partner health care delivery organization selection.
OMB and Public Comment
The Paperwork Reduction Act of 1980 requires clearanceh by OMB when standardized data collection from 10 or more respondents is to be performed on behalf of the Federal Government.
In June 2012, RAND submitted the Web and phone reporting forms, the phone interview protocol for the patient clarification process, and the protocol and questions to be used for the provider supplementation process. As required by the clearance process, notice was published in the Federal Register on Monday, September 10, 2012, and ran for 60 days. After revisions were made to the patient event reporting form, a second notice was published in the Federal Register on Thursday, June 6, 2013, and ran for 30 days. More than 100 sets of comments (45 with supporting documentation) were submitted by the public and reviewed by the research team.
Design Changes in Response to Public Comment
Some of the people who made public comments misconstrued the intent of the project to be the creation of a national government-run consumer reporting system. The team clarified that the intent was to create and evaluate a local prototype system—a potentially replicable model that health care organizations and other entities could use to elicit patient safety information directly from patients and caregivers with first-hand knowledge of their experience. Lessons derived from the testing of the prototype were intended to inform the design of future systems, with an emphasis on local adoption and replication rather than national deployment (which was seen to have had limited effectiveness based on 5 years of experience in the United Kingdom). Media and advocacy organizations took a generally supportive editorial view of the proposed reporting system, acknowledging the opportunity to enlist consumers in improving care safety.45
In response to the public comments and on the basis of the associated extensive review of the instrument, the research team revised the design of the hotline to include additional specific strategies to protect patient privacy, as well as protections for non-retaliation and for providers. No patient reports were to be shared with professionals or facilities unless the patient consented explicitly to such sharing and designated the facility or provider that should receive the information. All members of the research team would become members of the ECRI Institute PSO workforce. In compliance with the PSO statute, any material shared with the PSO regarding a report would include a header specifying that the included material is a patient safety work product (PSWP). Finally, in response to specific comments, the team made 19 revisions to the reporting form—changes in wording, the deletion of two items, and revisions of two items to be open-ended questions. There was no change in the survey burden or intent.
RAND submitted the final Section 508-compliant documents to AHRQ for submission to OMB on July 29, 2013, and received OMB approval on August 23, 2013.
Final Design Choices
Under the contract, AHRQ envisioned a passive solicitation strategy that relied on the participating health care delivery organizations to advertise the existence of the Web site and toll-free phone number through marketing efforts (e.g., posters, postcards, publication of the URL on their Web sites); the contract (and budget) did not envision the use of prompted solicitation methods such as post-discharge surveys or training a cohort of patients to record their observations about safety. Phone access was offered to accommodate non-English-speaking patients and families (the project budget did not allow for replicating the Web site in multiple languages).
The approach we adopted was to explore the challenges and opportunities of using consumer reports to drive health system-based patient safety improvements (rather than focusing on public accountability or patient safety research).
We used a variety of methods to ensure that the prototype reporting form was acceptable to patients and other caregivers, (e.g., we incorporated user perspectives on focus and terminology). We tested the wording of our data collection instruments to ensure that it was patient-friendly, but also that it tracked with the AHRQ Common Formats, enabling it to serve as a standard for reporting.
We also paid careful attention to creating a prototype structure that would make use of PSOs to allow health care organizations to analyze (and learn from) information contained in consumer reports without fear of liability risk.
We made these design choices prior to implementation, but we also realized that ongoing feedback from hotline users would be useful. We therefore created a voluntary and confidential post-report “usability” survey to collect such feedback.
Usability Survey
The 13-item post-submission usability survey was to be offered to consumers immediately after they completed a report online. It was intended to evaluate whether patients and caregivers found the hotline easy to use, effective, and efficient and whether they were satisfied with their hotline experience.
The survey was a modified Lewis Post-Study System Usability Questionnaire,46 with minor edits to make it relevant to the hotline (e.g., we replaced “this system” with “hotline” and added several questions). Completing the survey was estimated to take 5 minutes or less. The reading level of the survey was fourth grade, seventh month. The Operations Manual presented in Appendix C contains additional information about the survey, as well as the survey itself.
The Final Reporting Form
The final prototype patient reporting form has several innovative qualities that give patients options about the use of the information they provide and that allow for the collection of information specific to the concern being reported:
- The reporting form gathers information about the patient safety concern in both an open-ended narrative format and through a structured set of questions.
- The reporting form has a modular construction, and screener questions allow patients and caregivers to answer only those questions that pertain to their concern. The use of screener questions within a modular format allows the form to “drill down” to gain specific information based on the type of concern reported, as well as standard information collected for all concerns.
- Reports can be made by patient or by proxy (i.e., family member or other caregiver).
- The person making the report has the options of anonymity and confidentiality.
- The person making the report has the option of whether or not to allow followup.
- The patient or caregiver can control the sharing or accessing of the information within the health care delivery organization.
- The patient or caregiver has the option of identifying a particular clinician or health care facility.
Overview of the Patient Event Reporting Form Content
The final reporting form can be used with either the Web or the phone. It was constructed in modular form to capture key information about the event and the circumstances surrounding it in the following modules:
- Module 1: Introduction
- Module 2: Description of your safety concern
- Module 3: Mistake
- Module 4: Negative effect
- Module 5: Contributing factors, changes in care, discovery, and reporting
- Module 6: Patient and clinician/facility information
The modular format facilitates the input of information by providing some guidance, while also allowing patients/caregivers to skip around and answer only those questions they are able to answer. The reporting form is formatted with skip patterns to allow for reporting concerns that are considered medical mistakes, negative effects, or both.
With the required consent language, the event reporting form is at the eighth-grade reading level and scored an 8.0 on the Flesch-Kincaid reading scale,i with 4 percent passive sentences. Without the consent language, the reading level of the reporting form is seventh grade (7.7 on the Flesch-Kincaid reading scale).
We describe the contents of each module briefly below. The Operations Manual (Appendix C) contains the Web and phone versions of the event reporting form and includes a more detailed description of the form.
Module 1: Introduction
The introduction module provides information about the purpose of the reporting form, the time required to complete it, and other requirements and asks some questions pertaining to informed consent and the age of the patient or other person making the report. It includes a definition of the types of safety events that are appropriate for reporting to the hotline. It also advises patients and caregivers that other types of concerns, such as complaints about amenities (e.g., food or parking) or grievances that are not related to the safety of care, should be reported through other systems.
Module 2: Description of Your Safety Concern
This module contains a set of open-ended questions designed to obtain a narrative description of the patient safety concern. Examples of the questions are shown in Figure 2.1. The open-ended questions are followed by a series of questions with structured response elements about what happened, when and where it occurred, whether there were negative effects, and the type of such negative effects. A pop-up box provides definitions of the terms used.
Figure 2.1. Sample Open-Ended Questions in the Patient Event Reporting Form
|
Please tell us in your own words about the safety concern. Then we will ask some specific questions to make sure we understand what happened. |
Modules 3 and 4: Mistake and Negative Effect
A structured set of questions enable patients and caregivers to report about two types of safety events: (1) a suspected medical error or mistake—whether or not that medical error was associated with harm or injury; and (2) negative effects related to health care (e.g., harm, injury, adverse event). The patient or caregiver is asked whether he or she believes a doctor, nurse, or other health care provider made a medical mistake or error in the patient’s care; whether there was a negative effect; or whether he or she does not know if there was a mistake or a negative effect. If the respondent does not know whether the patient experienced a medical error or mistake or a negative effect, the form allows him or her to skip ahead to answer other questions (e.g., consent to share the response, contributing factors).
A patient safety event taxonomy using drill-down (multilevel) menus is embedded in the form. The prototype contains two top-level categories, each of which has a drill-down list of subcategories from which patients or caregivers select items to describe the mistake and/or negative effect experienced:
- Mistake
- Related to medicine
- Related to test, procedure, or surgery
- Related to pregnancy or childbirth
- Related to a diagnosis or advice from a doctor, nurse, or other health care provider
- Related to poor cleanliness or poor hygiene
- Related to something else, or more than one mistake
- Negative effect
- Related to medicine
- Related to test, procedure, or surgery
- Related to pregnancy or childbirth
- Related to a diagnosis
- Related to advice
- Related to unclean or unsanitary care
- Related to something else, or more than one negative effect
- Type of negative effect
- Physical
- Emotional
- Both
Module 5: Contributing Factors, Changes in Care, Discovery, and Reporting
Module 5 asks about the patient or caregiver’s perception of contributing factors. The respondent is given the option of describing in his or her own words the factors that might have contributed to the safety event; he or she is also asked whether each of a limited list of potential factors might have contributed. Included in this list are those factors that are plausibly directly observable by a patient or caregiver and are considered valid and reliable based on prior testing (e.g., prior surveys such as the CAHPS) or have been used in other patient safety reporting instruments. The list was not designed to be comprehensive; rather, it offers the most common responses. The form also offers opportunities for open-ended responses on many questions so that future versions can be refined. This section of the reporting form also asks whether the patient reported the event to a health professional, manager, or other person.
Module 6: Patient and Clinician/Facility Information
This module offers the patient or caregiver the option of identifying the health care personnel involved and providing contact information. It asks whether the patient or caregiver would like to grant consent for the report to be shared with a contact person at the facility, (e.g., an administrator or patient safety officer, or with individual doctors, nurses, or other health care professionals identified by the person making the report). The patient or caregiver has the option of recording the report anonymously or including his or her name and, if the respondent is a caregiver, his or her relationship to the patient. This module also asks whether the research team may contact the patient or caregiver for more information about the report. Finally, the form asks for demographic information about the patient and contact information about any clinician/facility involved.
Designing a Web-Based Data Collection Platform
The hotline prototype is Web-enabled. Access is controlled based on the role of the user (e.g., hotline administrator) and also on the specific types of data being collected. The Web interface captures the details of patient-reported safety events.
System Processing Requirements
The hotline employs a Microsoft Technology platform, including the latest operating system and software. It utilizes Microsoft Windows 64-bit 2008 R2 servers, Microsoft SQL Server 2012 64-bit, and ASP.NET.Architecture. It uses a well-known Web server architecture, in which a front-end tier (a Web browser) communicates with a back-end tier (a database) through a Web server tier. The minimum requirements on a single server are shown in Table 2.1.
Table 2.1. System Processing Requirements
| Operating system: | Microsoft Windows 64-bit 2008 R2 servers |
| Database: | SQL Server 2012 64-bit |
| Web server: | IIS 7 |
| Security: | Secure sockets layer (SSL) certificate |
The prototype utilizes Hypertext Transfer Protocol Secure (HTTPS), which is a combination of the Hypertext Transfer Protocol with the SSL protocol to provide encrypted communication and secure identification of a network Web server. An SSL certificate was procured by ECRI Institute to establish this secure transfer of data. The prototype supports the current and previous versions of Internet Explorer.
Telephone
Individuals may also access the hotline via the designated toll-free phone number. A phone-intake administrator (in the case of the prototype, a member of the research team) guides the person making the report (in either English or Spanish) by accessing the Web site and entering a new event. The patient or caregiver’s responses are entered in the first person.
Web
The hotline Web site was tested in a “staging” environment prior to deployment. All testing steps were intended to ensure that the Web site would be usable and efficient for all users. Functionality testing was conducted to ensure proper functioning of the Web site, survey skip logic, and decision support. Integration/usability testing was conducted to ensure that users of the system would be able to interface in a proper and efficient manner pertaining to workflow. Editorial content testing was conducted to confirm correct spelling and punctuation. Performance testing was completed to ensure that the database is properly architected and that the Web site code is efficiently written. Regression testing was performed along with functional testing to ensure that any changes made or “bugs” fixed did not introduce new problems. A Section 508 compliance review was conducted to ensure that the Web site is accessible to people with disabilities.
A more detailed description of the functional and nonfunctional requirements for operating the hotline is given in the Operations Manual in Appendix C.
Protocols for Data Collection, Processing, and Sharing
The protocol for processing hotline reports was designed to address several related priorities. First, the reports were to be screened for ongoing risks of harm that require immediate action or escalation, assuming the patient or caregiver has provided sufficient identifying information and has given permission to be contacted. This issue requires timely review by physician members of the research team. Second, we sought to ensure the confidentiality of respondents and personally identifiable information about care providers. Third, to clarify areas of a report that were confusing or ambiguous (e.g., degree or nature of harm, patients’ or caregivers’ attribution of causes, persistence of symptoms), the research team needed a process to elicit clarifying questions with an initial screen by a member of the research team as well as physician review. Fourth, a process for sharing information with key contacts at the partner health care organizations was needed to ensure that the information was received in time for the organization to conduct its own analysis and respond within the CMS regulatory timeline for addressing potential grievances. Figure 2.2 provides an overview of the steps for processing hotline reports. The steps are described below.
Figure 2.2. Overview of the Steps to Process a Hotline Report
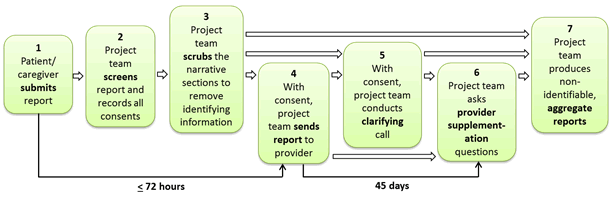
Step 1: Patient or Caregiver Submits Report
In Step 1, the hotline receives information about safety concerns through the reporting form (from the secure Web site or the toll-free phone number).
Before a patient or caregiver can answer questions about a safety concern, he or she is screened for age (a respondent must be at least 18 years old). Next, the respondent reads (or is read over the phone) text describing the types of safety concerns that should be reported to the hotline, the types of concerns that should not be reported to the hotline, and the types of information patients or caregivers may need access to in order to describe their safety concerns (e.g., month and year of the event, where the event occurred). Respondents must indicate that they understand this information (those using the Web-based form must click “Accept”), and they are then asked to answer the questions in the reporting form, as described earlier. The Operations Manual in Appendix C contains the full content of the Web-enabled form.
Back-end administrators (referred to as SuperUser – Administrators) process the reports after intake and collect additional information (where possible) from the health care delivery organization.
Step 2: Research Team Screens Report and Records All Content
Once a report is submitted, a member of the research team screens the report to confirm that it meets the inclusion criteria. During the screening process, the team member also notes whether the patient or caregiver consented to receive a followup call, whether permission was granted to share the report with the named provider, and whether the consumer’s name and contact information can be shared with the provider when a summary is sent. All activities are documented in an Excel tracking spreadsheet for administrative purposes (see the Operations Manual in Appendix C).
Step 3: Research Team Scrubs the Narrative Sections to Remove Identifying Information
To safeguard confidentiality, a research team member scrubs the narrative sections provided by the consumer, removing identifying information, such as names of people and institutions. Answers to questions that explicitly request identifying information, however, are not scrubbed. Complete instructions on how to audit or scrub a report, which prepares it for being shared, are given in the Operations Manual (Appendix C). The screening and auditing process is to be completed within 72 hours of receipt of the report.
Step 4: With Consent, Research Team Sends the Report to Provider
If the patient or caregiver consents to having the report shared with the relevant health care facility or provider, a PDF file of the scrubbed report is uploaded to a secure Web site that is accessible to the named provider. This is done within 72 hours to minimize delays and to enable the health care delivery organization to follow up with the patient or caregiver. Notification to the relevant health care facility or provider is sent via email, with a link to the report on the secure Web site.
Step 5: With Consent, Research Team Conducts a Clarifying Call
As part of Step 5, a physician on the research team reviews the report to determine whether there are any concerns or areas that require clarification. If clarification is required, a followup call is made to the person who made the report. The additional information elicited through the clarifying call is uploaded to the secure Web site if the patient or caregiver gives permission. The revised, clarified report is uploaded to the secure Web site, and the relevant health care organization or provider is notified via an email with a link to the clarified report. The Operations Manual (Appendix C) provides more information on the clarification call and gives examples of the types of questions asked.
Step 6: Research Team Asks Provider Supplementation Questions
Within 45 days of sharing the report with the named provider (Step 4), the research team reaches out to the health care delivery organization or facility with a series of questions about what the organization did with the patient-reported information. For example, the health care organizations participating in the pilot project were asked whether they were able to match the patient event report with any internal source of information (e.g., adverse event report, patient complaint) and what actions they took (e.g., conducting a root cause analysis [RCA], reaching out to the patient) after having received the information to improve patient safety within the organization. These questions are included in an administrative intake page linked to each report and are referred to as Module 8 questions (see the Operations Manual, Appendix C).
The sharing process is documented in an Excel spreadsheet. The research team documents the report identification number; the relevant health care organization; the date submitted; the date sent to the facility/provider; the date the clarified report was sent, if applicable; the date when the followup supplementation call is due (e.g., 45 days after receipt of the initial report); and the date on which the supplementation process with the relevant health care facility or provider was completed.
Step 7: Research Team Produces Non-Identifiable Aggregate Reports
Both before and after completing the clarification process, a physician member of the research team reviews and classifies each event according to the AHRQ Common Formats event type, harm scale, and duration of harm. The clinician also comments on the event’s preventability and contributing factors. The same reviewer classifies the initial and (if completed) clarified report. For research and summary purposes, the hotline can generate non-identifiable aggregated reports on the types of events reported.
With each report, progress through the seven processing steps is tracked in an administrative page linked to the report. The administrative page can be viewed by the research team but not by the patient or caregiver who made the report (details are outlined in the Operations Manual, Appendix C).
Clarifying Legal and Regulatory Protections for the Data
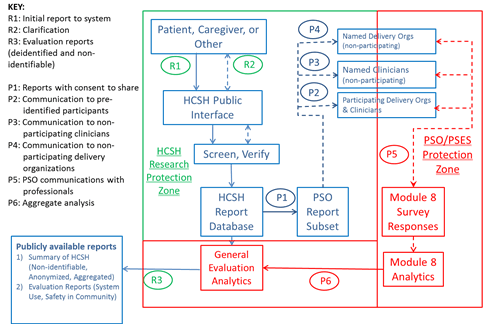
The research team consulted with legal counsel for AHRQ and the Department of Health and Human Services (HHS) to develop a clear and detailed understanding of the legal protections applicable to information provided to the hotline by patients and caregivers (see Figure 2.3). At least two forms of legal protection are applicable: First, the confidentiality provisions of the statute that created AHRQj requires that information obtained in the course of AHRQ-supported activities that identifies individuals or establishments can be used only for the purpose for which it was supplied. Information that is obtained in the course of AHRQ-supported activities and that identifies an individual may be published or released only with the consent of the individual who supplied the information or is described in it. There are civil monetary penalties for violation of the statute. The research team and AHRQ also determined that there is currently no requirement for destruction of the research data. However, reuse of the data would be allowed only for analyses consistent with the original purpose for which the data were collected. Second, the Patient Safety and Quality Improvement Act of 2005 establishes a framework with which hospitals, doctors, and other health care providers may voluntarily report information to PSOs, on a privileged and confidential basis, for the aggregation and analysis of patient safety events.k
The information in the hotline database is considered confidential research data. Under AHRQ’s authority to protect such data, this information cannot be publicly disclosed. If the consumer provides permission, the reported information can be sent to any named health care delivery organization or clinician. These organizations and clinicians can choose to analyze this information within a patient safety evaluation system (PSES) and can match the information to, for example, adverse event reports made by their health care staff. The information is treated as PSWP and cannot be publicly disclosed. The patient- and caregiver-reported information held by the hotline is kept in a database separate from the data provided by health care organizations to the PSO, in order to prevent disclosure of PSWP. The research team may produce aggregate, non-identifiable summaries and advisories. Once this research project is completed, the hotline database will be retained within the PSO.
Figure 2.3. Hotline Data Flows and Legal Protections
Summary
Through the design and development process, the research team identified the following key findings and attempted to address them in the development of the prototype. First, a number of design choices must be taken into consideration in developing a patient safety reporting system for consumers, including acceptability to patients and caregivers, awareness of the obligations created by patient reporting, and the choice of an outreach and marketing strategy. In addition, because health care organizations have existing requirements for responding to complaints and grievances—including those in State and Federal law—the consumer event reporting system must be in line with these requirements. Second, language is important in developing a reporting form for consumers. The patients and caregivers with whom we spoke struggled to understand the language and structured responses in the AHRQ Common Formats, which were developed to standardize reporting by health care professionals. However, there are some patient safety terms that are well understood by patients and caregivers, and these should be used in event reporting forms for patients. Third, encouraging narrative through the use of open text boxes at the beginning of a reporting form allows patients and caregivers to tell their entire story in their own words before answering structured questions. Both narratives and responses to questions are meaningful, and encouraging narrative responses may encourage consumers to finish their reports, even if the following structured items do not appear to be responsive to their specific experiences. Fourth, because patients and caregivers fear that reporting may have a negative impact on their future care, sufficient patient protections need to be in place and must be communicated early and well to patients and caregivers. Focus group participants told us that patients and caregivers are also skeptical about the impact that their reports will have on the health care system, so marketing materials should explain how the information will be used to improve patient safety at health care organizations and should provide assurances about the importance of respondents’ contributions.
e The patient’s rights provisions of CMS Conditions of Participation are found at 42 C.F.R. 482.13.
f Materials that are not gathered to be reported to a PSO and are not actually transmitted to a PSO by a health care organization do not qualify for the privilege and confidentiality protections of the Patient Safety and Quality Improvement Act of 2005. A significant amount of data remains outside the “patient safety work product” definition.
g For more information on AHRQ’s PSO program and the Common Formats, visit https://pso.ahrq.gov/common.
h “Clearance” is the term used for the process of obtaining approval from OMB for federally sponsored data collections (see www.hhs.gov/ocio/policy/collection/infocollectfaq.html, accessed July 27, 2015).
i The Flesch-Kincaid reading scale is a tool designed to test how difficult a reading passage is to understand. It has become a standard readability formula used by U.S. government agencies.
j The Healthcare Research and Quality Act of 1999, Pub. L. 106-129, codified at 42 U.S.C. 299c-3(c).
k The Patient Safety and Quality Improvement Act of 2005, Pub. L. 109-41, codified at 42 U.S.C. 299b-21-b-26.



