In 2016, AHRQ awarded a contract to develop methods and estimate the incremental inpatient financial costs and additional inpatient mortality associated with 10 selected hospital-acquired conditions (HACs):
- Adverse Drug Events.
- Catheter-Associated Urinary Tract Infections.
- Central Line-Associated Blood Stream Infections.
- Clostridium difficile Infections.
- Falls.
- Obstetric Adverse Events.
- Pressure Ulcers.
- Surgical Site Infections.
- Venous Thromboembolism.
- Ventilator-Associated Pneumonia.
The result is the development of 10 specific incremental cost estimates and 10 specific additional mortality estimates for these selected HACs. These data can be used in future national and other estimates of the total additional incremental inpatient financial costs of these HACs, and the total additional inpatient mortality associated with these HACs.
Contents
Summary
Introduction
Need for New Measurements
Data and Methods
Systematic Review Process
Identification
Screening
Evaluation
Data Extraction and Harmonization
Meta-Analysis
Results
HAC Specific Considerations
Adverse Drug Events
Catheter-Associated Urinary Tract Infections
Central Line-Associated Bloodstream Infections
Falls
Obstetric Adverse Events
Pressure Ulcers
Surgical Site Infections
Ventilator Associated Pneumonia
Venous Thromboembolism
Clostridium difficile Infections
Discussion
Limitations
Competing Risk and Double Counting Issues
Underlying Data Concerns
Opportunities for Future Research
Appendix A. PubMed Search Criteria
Appendix B. Excess Mortality Calculations
Appendix C. Meta-Analysis Citation List
Appendix D. Key Study Characteristics
Appendix E. Forest Plots
References
List of Exhibits
Exhibit 1. Threats to validity and consistency of current HAC estimates
Exhibit 2. Methods overview for estimating HAC-associated cost and mortality
Exhibit 3. HAC definitions based on AHRQ Common Formats for Surveillance
Exhibit 4. Dimensions and criteria for inclusion
Exhibit 5. Format variations in cost reporting and conversion strategy
Exhibit 6. Comparison of random and fixed effect meta-analysis models
Exhibit 7. Summary of meta-analysis additional cost estimates
Exhibit 8. Summary of meta-analysis excess mortality estimates
Exhibit 9. Comparison to 2010 AHRQ estimates
Summary
Despite advancements in infection control and injury prevention, hospital-acquired conditions (HACs) continue to have a high financial burden on the health care system and contribute significantly to inpatient morbidity and mortality in the United States. Multiple Federal initiatives in patient safety highlight the need for better understanding of additional cost and excess mortality due to HACs, including the U.S. Department of Health and Human Services (HHS) National Quality Strategy1,2 the National Action Plan to Prevent Health Care-Associated Infections: Road Map to Elimination,3 and the National Action Plan for Adverse Drug Event Prevention.4 Several efforts are underway at the Agency for Healthcare Research and Quality (AHRQ),Centers for Medicare & Medicaid Services (CMS), and the Centers for Disease Control and Prevention (CDC) to target and reduce the incidence of HACs through implementation of evidence-based strategies and better measurement and reporting. Federal agencies are leading significant efforts in these areas: for example, the public-private Partnership for Patients (PfP),5 AHRQ's Comprehensive Unit-Based Safety Programs (CUSP),6 National Scorecard for HACs,7 Quality and Safety Review System (QSRS),8 and CMS' HAC Reduction Program related to payment reform.9
The goal of this project, conducted by NORC at the University of Chicago (NORC) in partnership with Emory University, on behalf of AHRQ, is to estimate the excess cost and mortality associated with 10 HACs being targeted for improvement:
- Adverse Drug Events (anticoagulants, opioids, and hypoglycemic agents)
- Catheter-Associated Urinary Tract Infections
- Central Line-Associated Bloodstream Infections
- Falls
- Obstetric Adverse Events
- Pressure Ulcers
- Surgical Site Infections
- Ventilator Associated Pneumonia
- Venous Thromboembolism
- Clostridium difficile Infections
In this report, we present the findings of our literature review of empirical research on additional cost and excess mortality for the 10 HACs listed above. For this review, we used 69 studies in 20 individual meta-analyses to estimate the additional cost and excess mortality associated with each of the HACs on a per-HAC basis. Estimated added costs across all HACs ranged from $600 to $48,000 per case, while excess mortality estimates ranged from 5 deaths per 1,000 cases to 150 deaths per 1,000 cases. These values will assist Federal efforts to track progress on improving patient safety and eliminating HACs.
Introduction
The goal of this project is to generate updated, robust estimates of the excess costs and mortality associated with 10 hospital acquired conditions (HACs) being targeted by the the Department of Health and Human Services (HHS) part of the current Partnership for Patients program (PfP).10 HACs are conditions that are not present upon hospital admission but rather are acquired during the period of hospitalization. They can stem from diagnostic or treatment errors (e.g., failure to follow antibiotic protocols); medical injuries or adverse events; or exposure to pathogens, such as Clostridium difficile. The consequences of HACs can be serious for patients, ranging from increased length of hospital stay to worsened health outcomes or unexpected mortality. Medical errors and HACs affect all age groups, from neonates and mothers during labor and delivery to surgical patients to elderly patients—all vulnerable during a hospital stay. Many HACs can be effectively addressed and prevented through training, adherence to evidence-based treatment guidelines, and hospital best practices, but only if the HACs are first properly measured and understood.11 Of particular interest to AHRQ are 10 HACs:
- Adverse Drug Events (ADE)
- Catheter-Associated Urinary Tract Infections (CAUTI)
- Central Line-Associated Blood Stream Infections (CLABSI)
- Falls
- Obstetric Adverse Events (OBAE)
- Pressure Ulcers
- Surgical Site Infections (SSI)
- Venous Thromboembolism (VTE)
- Ventilator-Associated Pneumonia (VAP)
- Clostridium difficile Infections (CDI)
Since the Institute of Medicine's landmark publications To Err Is Human (1999) and Crossing the Quality Chasm: A New Health System for the 21st Century (2001) revealed the extent of preventable medical errors, significant effort has been directed at decreasing the incidence of these adverse events and improving patient safety.12,13 Following passage of the Patient Protection and Affordable Care Act (ACA), HHS reestablished patient safety as a national health care priority area and sought to eliminate HACs through multipronged policy- and program-based approaches. These approaches are detailed in the AHRQ-led National Quality Strategy,1,2 the National Action Plan to Prevent Health Care-Associated Infections: Road Map to Elimination,3 and the National Action Plan for Adverse Drug Event Prevention.4 These documents articulate the goals, priorities, and measures for improving quality and decreasing the incidence of HACs, given their significant risks to patient health and safety. AHRQ and CMS, in particular, are leading this charge.
AHRQ and CMS are partnering with other HHS agencies to conduct a range of activities to address HACs, hospital-acquired-infections (HAIs), and medical errors specifically, as well as patient health and safety more generally. For example, both AHRQ and CMS encourage the practice of evidence-based quality and infection control—including through a national technical-assistance program implemented by CMS-funded Quality Innovation Network-Quality Improvement Organizations (QIN-QIOs), and through the Community-Based Care Transitions Program (CCTP), which aims to reduce hospital readmissions for high-risk Medicare beneficiaries by more effectively managing their care and transitions.14 The goals of the PfP program are to improve safety in acute-care hospitals, and achieve a 20 percent reduction in HACs and a 12 percent reduction in 30-day readmissions as a population-based measure (readmissions per 1,000 people) from 2014 to 2019.15,16
CMS is also using payment model restructuring to encourage HAC reductions as part of payment reform efforts. Beginning in 2008, CMS identified the HACs (e.g., CAUTI, falls and trauma, surgical site infection) for which certain types of hospitals would be subject to mandatory reporting with payment penalties (i.e., they would not be paid for services related to treating those HACs).17 In 2010, the ACA formally established the Hospital-Acquired Condition Reduction Program, offering incentives for HAC reduction; however, beginning in 2015, the ACA required CMS to reduce payments to hospitals performing in the bottom 25 percent on HAC-related quality measures.18 The accuracy of these measures in capturing HACs has therefore become a key factor for both patient health and hospital payments.
AHRQ is pursuing a variety of measurement-related activities for improving patient safety and outcomes, including for specific HACs. For example, the AHRQ National Scorecard on Rates of Hospital-Acquired Conditions leverages data from the Medicare Patient Safety Monitoring System (MPSMS).19 In its 2014 final report based on MPSMS and other data, AHRQ noted a decrease between 2010 and 2014 that corresponded to reductions of 17 percent, or 2.1 million HACs. Furthermore, the report finds significant improvements in additional cost and excess mortality: the reduction in HACs translated to a savings of 87,000 patient lives and $19.9 billion.20
Despite these achievements, HACs continue to have a significant financial and human cost, and efforts to quantify and reduce HACs are an important ongoing effort for HHS. There is broad consensus on the importance of accurately measuring the incidence and impact of HACs from the perspective of patient health and the role that such measures play in improving patient safety through performance-based payment reform. In particular, additional scrutiny is being applied to the measurement of HACs and their impact on inpatient hospital costs and mortality. For example, AHRQ is developing and implementing a successor system to MPSMS: the Quality and Safety Review System (QSRS), which will be described in the section that follows. The QSRS will expand the number of HACs being targeted and include additional data types, sources, and capabilities to better measure HAC incidence and impact.21,22
Need for New Measurements
In addition to the patient safety implications, accurate measurement of HAC incidence and severity has direct consequences for hospital payments as part of the transition from fee-for-service to pay-for-performance reimbursement. Until recently, a combination of the MPSMS measures, ICD-9 codes (now ICD-10), and AHRQ Patient Safety Indicators (PSIs) were used to inform public health surveillance and reimbursement decisions. However, PSIs have been criticized for their reliance on claims rules instead of clinical criteria for defining HACs.23 With greater availability of structured electronic health record data, it is now more feasible to incorporate clinical characteristics (e.g., laboratory test results) into patient safety event descriptions. Because of increased access to clinical data and questions about whether existing HAC definitions can fully capture the incidence of a given HAC, AHRQ has revised its definitions to incorporate clinical features into formerly claims-based definitions.
These changes take the form of AHRQ’s new patient safety surveillance system—the QSRS that was mentioned above. It will replace the MPSMS and attempt to address some of the prior measurement limitations by 1) simplifying event descriptions, 2) expanding the scope of adverse events collected, and 3) creating consistency across other patient safety data collection initiatives (e.g., the AHRQ Common Formats for Surveillance definitions for hospital-acquired infections are based on the CDC’s National Healthcare Safety Network definitions).21,22 With these new definitions being used in QSRS and represented in the aforementioned PfP 2019 goals, it is important to update estimates of additional cost and excess mortality associated with HACs. These estimates can then inform patient safety and quality improvement efforts to measure success in reducing HACs and the burdens associated with them.
In spite of the new definitions used by QSRS—which will take time to be fully implemented and to be reflected in the literature—certain measurement challenges remain and must be taken into account when estimating and interpreting HAC prevalence. Exhibit 1 summarizes some of the often-highlighted threats to validity and consistency of current estimates.24,25
Exhibit 1. Threats to validity and consistency of current HAC estimates
- Definitions of HACs vary by data source (clinical vs. claims-based).
- Estimates derived from a subpopulation of patients with specific conditions or insurance coverage.
- Inconsistency in extent to which estimates of cost and mortality account for severity of HACs and interactions among different HACs.
- Estimates not based on systematically combining pertinent quantitative data from studies.
- Definitions of costs do not reflect actual additional incremental cost to the hospital attributable to the HAC.
- Definitions of mortality do not reflect additional deaths associated with the HAC.
- Estimates not based on recent literature.
To address the need for updated estimates using the more recent QSRS-revised HAC definitions, AHRQ funded this study with the goal of producing valid, meaningful measures of additional cost and excess mortality. Below, we describe our systematic review of the literature, including our methods for identifying relevant literature on each HAC, and our search criteria for the clinical, cost, and mortality aspects of each HAC. We present our estimates of additional costs and excess mortality based on the meta-analysis and compare our estimates to prior estimates. Finally, we provide recommendations for additional research and describe challenges encountered and limitations to the findings.
Data and Methods
This section describes our approach (shown in Exhibit 2) of first reviewing the literature, then synthesizing the findings from the literature through meta-analysis, and finally producing estimates of additional costs and excess mortality for each HAC (Exhibit 2).
Exhibit 2. Methods overview for estimating HAC-associated cost and mortality
| Systematic Review Process | 1) Identification: Using key word searches, identify potentially relevant literature in publication databases and grey literature search engines. | → | 2) Screening: Review title and abstract to identify articles most likely to contain relevant cost and mortality data. | → | 3) Evaluation: Subject matter experts assess full text for appropriate HAC definitions, measurement of outcomes, and methodological soundness. |
| ↓ | |||||
| Data Extraction & Harmonization | 4) Secondary evaluation: Assess full text for cost and mortality definitions applicable to "in-hospital" adverse events and for outcomes that can be converted and synthesized into a single meta-analysis. | → | 5) Quality assessment: Assign "high," "medium," or "low" ratings to articles based on HAC definition, generalizability of study, and methods used to construct estimates and control biases. | → | 6) Data extraction & conversion: Extract cost and mortality estimates, and document study methodology and results; harmonize differing cost formats for use in the meta-analysis. |
| ↓ | |||||
| Meta-Analysis | 7) Construct dataset: Use Stata 14 meta-analysis package to create a dataset with attributable cost and mortality estimates from the literature. | → | 8) Analysis: Apply random-effects models using the DerSimonian and Laird method. | → | 9) Results: Produce overall cost and mortality estimates for each HAC. |
Systematic Review Process
The first stage of our work involved conducting a systematic review of published and grey literature for articles containing data on inpatient cost and/or mortality related to the HACs of interest. Our process is based on the PRISMA statement on systematic reviews and meta-analysis and includes three steps—identification, screening, and evaluation.26 Throughout the review process, articles were assessed for relevance by applying increasingly rigorous criteria at each successive step. The remainder of this section describes each step in more detail.
Identification
The first step in the systematic review process was to define potentially relevant literature for each HAC. We developed HAC-specific search criteria to search publication databases, including PubMed, Scopus, and grey literature search engines. We then conducted forward and backward searches on relevant literature (e.g., references, articles that cited the original search results) and supplemented these searches with articles identified from the reference list of prior meta-analyses and systematic reviews. This multipronged search strategy better ensured that we captured the most relevant literature for each HAC.
Each search string contained three distinct groups of search terms, designed to target different aspects relevant to our review: HAC definition, outcomes (cost and/or mortality), and inclusion/exclusion criteria.
For each HAC, we used medical subject headings and included related terms drawn from AHRQ’s recently developed Common Formats for Surveillance event description. AHRQ created the Common Formats for Surveillance as part of its effort to standardize HAC definitions and take into account both clinical and claims-based information in monitoring patient safety events. These definitions are intended to be applied by hospitals and patient safety officers in their surveillance for adverse events and form the basis for AHRQs transition from the MPSMS to QSRS. Exhibit 3 provides brief definitions for each HAC.
Exhibit 3. HAC definitions based on AHRQ Common Formats for Surveillance
| HAC | Common Formats for Surveillance Definitions |
|---|---|
| Adverse Drug Events (ADE) | An event in which administration of a medication results in harm to a patient. Included are adverse reactions during or following administration without any apparent incorrect action. |
| Catheter-Associated Urinary Tract Infections (CAUTI) | Infection of the urinary tract that occurs subsequent to insertion of an indwelling urinary catheter during the hospital stay. |
| Central Line-Associated Bloodstream Infections (CLABSI) | Infection of the blood stream that occurs subsequent to insertion or access of a central line or umbilical catheter during the hospital stay. |
| Falls | Fall during an inpatient admission, with or without injury, whether or not fall was assisted. |
| Obstetric Adverse Events (OBAE) | An adverse maternal or fetal outcome that occurs during labor and/or birth. It includes eclampsia, infection not present on admission, injury to other body part, and fetal or maternal death, among others. |
| Pressure Ulcers | A new pressure ulcer developed during a stay, or Stage 1 or 2 pressure ulcer(s) present on admission advancing to Stage 3 or 4, unstageable, or the development of osteomyelitis, tunneling, or fissure contiguous to any pressure ulcer. |
| Surgical Site Infections (SSI) | Infection that occurs prior to discharge and within 30 days following an inpatient operative procedure that involves any part of the body that is opened or manipulated as part of the procedure. |
| Ventilator-Associated Pneumonia (VAP) | Acute pneumonia caused by bacteria, viruses, or fungi among inpatients mechanically ventilated for at least 2 days prior to pneumonia diagnosis. |
| Venous Thromboembolism (VTE) | A deep vein thrombosis (DVT) or pulmonary embolism (PE) developing among inpatients after admission. |
| Clostridium difficile Infections (CDI) | An infection of the gastrointestinal tract, in patients 2 years of age or older, that was not present on admission. Infection is indicated by clinical confirmation (i.e., notation of diarrhea or “pseudomembranous colitis” in medical records) or laboratory confirmation (i.e., positive test results for CDI toxin A and/or B or toxin producing CDI organism found in stool sample). |
To develop search terms for cost and mortality outcomes, we focused search criteria on terms likely to produce articles on inpatient stays and methods that allow for calculating attributable or excess additional cost and excess mortality. Our initial inclusion/exclusion criteria limited results to original analyses of hospitalizations in the United States, articles published since 2000, and the English language. For some HACs where we found few studies of sufficient quality to include, we expanded the time frame to include pre-2000 data. A full list of search terms is provided in Appendix A. To be selected for review, articles needed to mention the HAC, include either inpatient cost or mortality, and meet the inclusion and exclusion criteria.
Screening
After article identification, a member of the NORC team assessed each article’s relevance to the HAC, cost, and mortality through a multistep process of categorization and review. First, citations of articles in the search results were categorized by HAC and outcome (i.e., cost or mortality) and saved in Mendeley, a citation manager application. We then imported the citations into Covidence collaboration software, which was used to assign reviewers and track the review process. Once the citations were in Covidence, at least one staff member screened the title and abstract of each article against the search and inclusion criteria to determine whether it would be included in the next stage of a full text review. As in the previous stage, articles had to involve primary or secondary analysis of data from U.S. hospitals and present cost and/or mortality related to one or more HACs. This screening was a necessary step to eliminate articles that were returned by the search but did not meet all the relevant criteria (e.g., non-U.S. data, community-acquired instead of hospital-acquired conditions).
Evaluation
The final step in the systematic review process was evaluation of the full text of remaining articles by a multidisciplinary team of experienced reviewers including epidemiologists, health economists, and clinicians. Two team members independently reviewed each article and provided an assessment on whether to include or exclude the paper from meta-analysis. In the event that the two reviewers provided differing assessments, a third reviewer made the final decision. Reviewers evaluated articles on three key dimensions for inclusions (Exhibit 4).
Exhibit 4. Dimensions and criteria for inclusion
| Category | Specific Criteria |
|---|---|
| General |
|
| Methodology |
|
| Applicability |
|
Since articles varied widely in the details they provided in the titles and abstracts, some articles passed the screening and were then excluded during full-text review for failing to meet general criteria (e.g., research conducted at a non-U.S. facility). However, the preponderance of full-text review focused on assessing the methodology and applicability of the studies to our research goals. Specifically, we excluded from the meta-analysis stage articles that met any of the following conditions:
- HAC definition did not approximate the Common Format for Surveillance definitions.
- Cost or mortality definitions did not approximate AHRQ’s definition.
- Population studied was not in an inpatient setting.
- Study design or methods were deemed inappropriate for calculation of attributable cost or mortality.
Data Extraction and Harmonization
Once the full text reviews were completed, we moved the studies into the data extraction stage. Members of the data extraction team conducted a close reading of the remaining articles and evaluated them based on the appropriateness of HAC definitions used, outcomes measured, and methods applied for use in our analysis. We excluded articles from analysis if the definition used for the HAC did not approximate the Common Formats for Surveillance definitions closely enough to be appropriate for the final HAC estimates. We scrutinized cost and mortality definitions for their applicability to “in-hospital” adverse events. We also evaluated how the outcomes were reported (e.g., differences among groups, raw or adjusted, odds ratio versus relative risk) and whether these could be harmonized with other studies for the meta-analysis.
After providing a quality assessment for each article, team members progressed to extracting cost and mortality parameters and documenting pertinent details from each study’s methodology and results. For mortality, we extracted the counts of HAC cases and controls, mortality rate or number of deaths for each group, and relative risk (RR) or odds ratio (OR) (if reported). If a study reported events for cases and controls, we calculated a relative risk from these values using the formula:
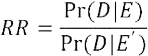
Probability of death among the cases (Pr D|E) is divided by the probability of death for the controls (Pr D|E’) to obtain the relative risk. When the RR was not reported or could not be calculated, we used the OR value to approximate RR, based on an assumption that the overall mortality rate was low.
Then, taking the relative risk—either as reported in the publication or as calculated based on case and control numbers or as approximated by the OR—we conducted meta-analysis with the log format of these values and the corresponding standard errors for mortality. In order to convert to excess mortality, we combined an underlying mortality rate of the at-risk population for the HAC with our pooled relative risk estimate from the meta-analysis, using the formula:
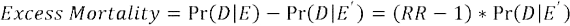
In the above formula, RR indicates the pooled relative risk estimate from our meta-analysis, and Pr(D|E’) represents the underlying mortality in patients at risk for the HAC. Excess mortality is reported as the number of deaths per case of HAC. Appendix B provides more details on excess mortality calculations and underlying mortality rates used.
For cost parameters, we extracted available information to calculate additional cost estimates and standard error for each study. In Exhibit 5, we list examples of formats for cost outcomes found in various articles
and how we decided to convert those estimates into similar figures for use in meta-analysis. All the cost values were adjusted to 2015 U.S. dollars using Producer Price Index for general medical and surgical hospitals.27 Final estimates of additional costs, similar to excess mortality, are presented as additional costs per case of HAC.
Exhibit 5. Format variations in cost reporting and conversion strategy
| Cost Reporting Format | Conversion Strategy | Assumption/Reference |
|---|---|---|
| Hospital charges | Cost = charge * 0.5 | Cost-to-charge ratio of 0.528 |
| Mean estimate for HAC and non-HAC group separately | Attributable cost estimate = Mean(HAC) – Mean(non-HAC) | Attributable cost is the adjusted mean difference between cases and non-cases |
| Standard deviation (SD) | Standard error (SE) = SD/SQRT(N) | |
| 95% confidence interval | SE = (upper confidence limit – lower confidence limit)/2/1.96 | |
| Median and interquartile range (presented as Q1 to Q3) | Mean = (Q1 + Median + Q3)/3 SD = (Q3 – Q1)/1.35 |
Higgins JPT and Green S (2011)29 |
| Median and range (presented as Min to Max) | Mean = (Min + 2 * Median + Max)/4 SD = (Max – Min)/6 |
Hozo et al. (2005)30 |
| SE for HAC and non-HAC group separately | SE of attributable cost estimate = SQRT{SE2(HAC) + SE2(non-HAC)} | Only if cases and non-cases are independent samples and the sample size is large |
Meta-Analysis
After collecting and converting cost and mortality parameters for included studies, we conducted analysis using Stata 14 (StataCorp LP) meta-analysis packages. Specifically, we used the metaan command to generate attributable cost estimates and the relative risk for mortality estimates. We then calculated excess mortality based on the estimated relative risk and the underlying mortality of the at-risk population.31
We assumed that studies had enough in common to be incorporated in the meta-analysis for synthesis. Though studies are similar, they are not exactly the same; therefore, we allowed variation (heterogeneity) in effect estimates across studies due to factors such as study design type, analytic method, patient subpopulations, treatment standards, and geographic region in the United States. Thus, for analysis, we applied random-effects models using the DerSimonian and Laird method.32,33 Random-effect models assume that studies included in the meta-analysis are a random sample of the distribution of effects and allow the true effect to vary from study to study. This method weights studies based on the inverse of the sum of the variance estimated between studies and the individual sampling variance. In the event that no substantial heterogeneity is observed from the random-effects model, we would then apply a fixed-effects model, which assumes that all the included studies share one true effect size. More details on the differences between random- and fixed-effect meta-analysis models are provided in Exhibit 6.
Exhibit 6. Comparison of random- and fixed-effect meta-analysis models
| Random-Effect Model | Fixed-Effect Model | |
|---|---|---|
| Assumption | Studies were drawn from populations that differ from each other in ways that could impact on the treatment effect. | All studies shared a common true effect size. |
| Reason for effect size variation | Variation comes from random error within studies and true variation from one study to the next. | Variation comes from random error within studies. |
| Weight assignment | Large studies receive more weights than smaller studies. | Weights are balanced between large and small studies. |
Due to the requirement of using consistent format of parameter inputs in meta-analysis, we had to exclude some studies even though they reported cost and/or mortality outcomes. For example, we excluded studies that only reported attributable cost and significance level because we cannot generate a standard error for the attributable cost based on that information.



