David P. Baker, Anthony D. Slonim, and Patrice Weiss
Abstract
Background. A prevailing strategy for reducing patient liability claims is full disclosure. Research has shown that when health care professionals disclose their mistakes, payouts for claims against providers and the hospital are reduced. Therefore, it is important to understand how to include patients and families in the care team and how to communicate with patients about the risks and mistakes that can occur throughout the care process.
Methods. To explore these important issues, this project addressed two aims. First, we sought to identify adverse clinical events that are highly dependent on provider teamwork, require patients and families to be effective members of the team, and vary in terms of risk and liability (high risk, high liability; high risk, low liability; low risk, high liability; low risk, low liability). The resulting events created four distinct clinical situations in Labor and Delivery for studying the second aim. Specifically, we wanted to determine if patients, family members, and providers agreed about what kinds of information should be disclosed regarding each event and explore how communication should occur to mitigate risk and reduce liability. As this study was exploratory in nature, we used surveys, interviews, and focus groups to address these two aims.
Results. Our preliminary findings demonstrated that across the four obstetrical events (that varied in terms of risk and liability), there was far more agreement among patients and family members regarding what should be disclosed when an adverse event occurs as compared to clinicians. Type of event seemed to affect what failures clinicians indicated should be disclosed, while type of event had little effect on what failures patients and family members indicated should be disclosed.
Conclusion. Despite several limitations—including small sample size, the use of qualitative information, and application in just one clinical area—our work provides a starting point for further studies around disclosure and communication among clinicians, patients, and families about errors in medical care.
Introduction
Since the release of the Institute of Medicine (IOM) report To Err is Human, the argument that teamwork is essential for the effective delivery of health care has been undisputed.1 In 1999, Risser and colleagues demonstrated that teamwork breakdowns in the emergency department (ED) were a critical root cause of sentinel events at eight hospitals, costing approximately $3.50 per ED visit.2 Mann, Marcus, and Sachs 3 found that team training improved team performance in Labor and Delivery (L&D) and reduced the number of claims made against the L&D service at Beth Israel Deaconess Medical Center by 50 percent over a 3-year period. Arguably, team training has tremendous potential as a risk mitigation and liability reduction strategy because of its ability to improve care quality. To address this vital need, the Agency for Healthcare Research and Quality (AHRQ) and the Department of Defense (DoD) released TeamSTEPPS® (Team Strategies and Tools to Enhance Performance and Patient Safety) as a public domain resource to improve team performance and coordination of care within the national health system.4
In addition to improving teamwork, another prevailing strategy for improving care quality and reducing liability claims is full disclosure. Research has shown that when providers disclose their mistakes, payouts for claims against the doctor and the hospital are reduced. Many States now have medical disclosure laws that require physicians and health systems to disclose information related to the event to the patient and the patient’s family. In their MEDiC legislation, then Senators Clinton and Obama proposed that hospitals should receive grant money for being up front with patients and families after a medical error. They would then immediately negotiate compensation with the patient or patient’s family. The patient’s family would still be able to bring their case to court; however, research found that this was less likely to happen.5
A central tenet of improving teamwork in health care is that the patient and the patient’s family members are critical members of the team. The concept of patient-centered care and the growing implementation of patient-centered medical homes reinforce the important role patients and their family members play in the delivery of effective and efficient health care. Studies have shown increases in patient satisfaction and self-efficacy when patients and families are included in the care team.6,7 Therefore, from a quality, risk, and liability standpoint, it is important to understand how to include patients and families in the care team, how to equip patients and families to be effective team members, and how to communicate with patients and families about the risks and mistakes that can occur throughout the care process. Combined, inclusion of patients and family members in the care team and preparation through team training should yield better communication between providers and patients and therefore better care with reduced risk.
Research, however, has yet to specify how to include patients and their family members as part of the care team, how to train patients and their family members to be effective team members, what this training should consist of, when this training should occur, and what would be the result. Such work is critical in understanding how to foster better communication and ultimately teamwork between providers and their patients.
To begin to investigate these important issues, we sought to explore two aims. First, we wanted to identify clinical events that are highly dependent on provider teamwork, require patient and families to be effective members of the team, and vary in terms of risk and liability. Aim 1 was important because it yielded a set of events that could be used to explore issues related to provider disclosure and how patients and families can be active participants of the care team. Second, we wanted to ascertain how patient and family and provider communication should occur to mitigate risk and reduce liability. Aim 2 focused specifically on provider disclosure and patient, family, and clinician perceptions of what should be disclosed related to Aim 1 events.
Below, we describe the two sequential exploratory studies that were conducted to address each aim. Results from Aim 1 were used specifically to investigate Aim 2. For each study, we present the methodology employed and the results and findings.
Research Study 1
The goal of Study 1 was to identify four clinical events that are highly dependent on provider teamwork, require patients and families to be effective members of the team, and vary in terms of risk and liability. Once the events were identified, we used a series of interviews to identify the critical breakdowns that can occur to yield each event. The resulting events served as a set of stimuli that could be used to explore Aim 2.
Methods
To identify events, two activities were performed. First, we reviewed a report by the RAND Corporation 6 that identified adverse events in which team performance was critical.8 RAND was contracted by AHRQ to investigate where indicators of teamwork were most evident in the clinical environment. Using a modified Delphi technique, RAND identified 11 events in different clinical settings that required high degrees of teamwork. More details about this study are presented in the next section.
We used the resulting RAND events to develop a survey. The survey was used to collect ratings from several clinicians regarding their perceptions of risk, liability, and requirements for teamwork associated with each event. These ratings were used to select a subset of events that could be used to explore teamwork, communication, and liability issues under Aim 2. Each of these steps is described in more detail below.
RAND Report
The purpose of the RAND investigation was to identify adverse outcomes for which teamwork is a critical factor. RAND relied on a modified Delphi method in which a multidisciplinary group of clinical experts rated the relationship between teamwork and a pre-selected list of clinical outcomes in L&D, acute myocardial infarction (AMI), and surgery. RAND identified the following events in L&D: birth trauma, injury to neonate–C-sections; birth trauma, injury to neonate–vaginal birth; uterine rupture; maternal death; and intra-partum fetal death of full-term infant. In the surgical area, RAND identified the following adverse patient outcomes: failure to rescue; foreign body left in during procedure; and mortality despite low-mortality Diagnosis Related Groups (DRG) code. However, for the AMI domain, RAND was unable to recommend any outcome measures related to teamwork.
Survey Development and Administration
Based on the RAND report and discussions among our research team, we decided to focus our research on identifying obstetrical events that required teamwork and varied in terms of risk and liability. L&D events can be high risk and often yield the highest liability payouts.
To explore the characteristics of the seven L&D events identified in the RAND report, we developed a short survey that listed these adverse obstetrical events and asked clinical experts to rate each regarding the degree of risk, likelihood of liability, and the requirement for teamwork using 5-point Likert scales. Because the RAND report yielded only seven adverse outcomes (i.e., a small number), two physicians knowledgeable about obstetrical care identified 11 additional events to include on the survey. The goal of this activity was to ensure that the survey had a variety of events that varied along the risk, liability, and teamwork dimensions. One expert was an obstetrician, and the other was a pediatric critical care physician (the final survey instruments are available upon request from the authors).
Participants
The survey was completed by a convenience sample of 10 clinicians from the Mother-Baby Unit of a large southeastern health system. Respondents had an average of 5.7 years of experience as an obstetrician. Of the 10 participants, four were residents and six were attending physicians. Three of the participants had previously participated in team training; the other seven had no prior team training experience.
Results
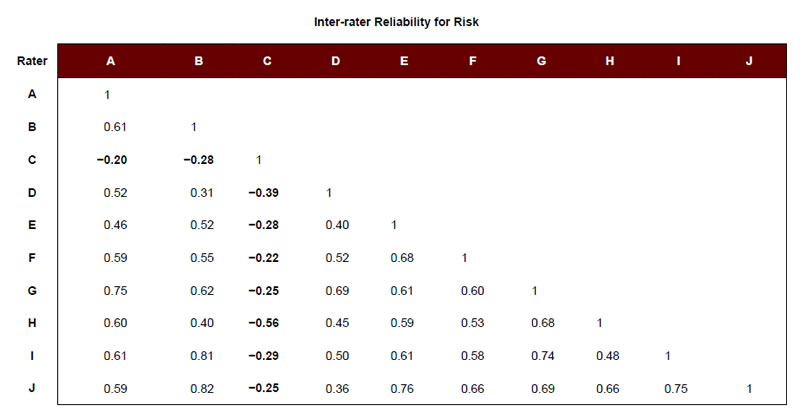
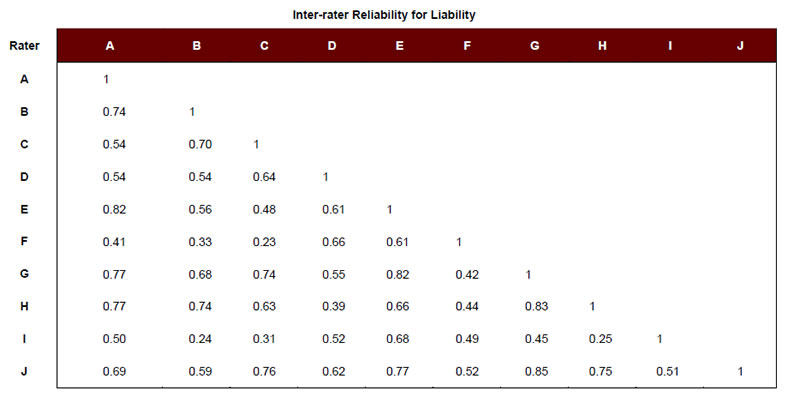
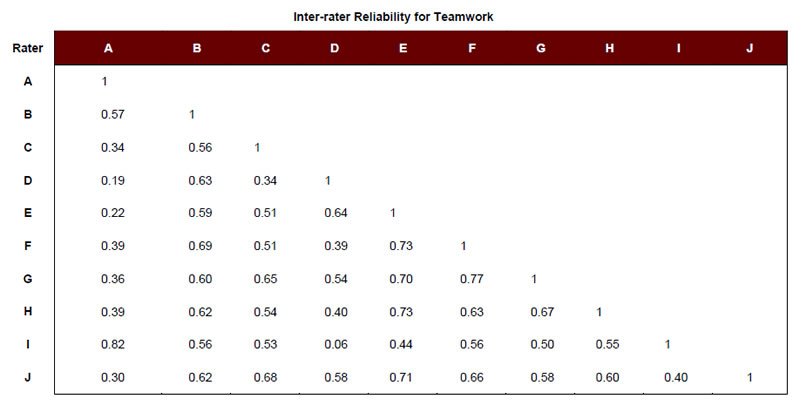
To identify a set of adverse obstetrical events that could be used to study Aim 2, we used the survey responses of the 10 clinicians to create average risk, liability, and teamwork scores for each event (Table 1). Each event was then categorized as “high” or “low” on each particular attribute (i.e., risk, liability, and teamwork), using the respective mean rating across events. For example, the mean rating for risk across adverse events was 2.47. Events scoring above 2.47 were considered to be “high” risk, and those scoring below 2.47 were considered “low” risk. We also examined the degree of inter-rater agreement across respondents to see if our clinical experts agreed regarding the relative risk, liability, and teamwork associated with each event. Except in the case of Respondent C regarding the risk ratings, reliabilities were observed to be moderate to high (Appendix A).
Next, because a primary objective of this research was to focus on events (regardless of their degree of risk or liability) that required teamwork, we identified those events that received high scores in terms of teamwork (i.e., above the mean teamwork rating). Events that scored high in teamwork were then allocated to four different categories of risk and liability: high risk, high liability; high risk, low liability; low risk, high liability; and low risk, low liability (Table 2). The study team then reviewed these events and selected one event to represent each category (Table 2). The final, selected events were: Shoulder Dystocia, Post-Partum Hemorrhage, Intra‑Partum Fetal Death due to Group B Strep, and Unplanned Return to L&D or Operating Room (OR).
Last, we interviewed 12 clinicians about the common failures that can occur leading to each event. Specifically, we interviewed a sample of three clinicians about the failures that can lead to each of the four events: Shoulder Dystocia, Post-Partum Hemorrhage, Intra-Partum Fetal Death due to Group B Strep, and Unplanned Return to L&D or OR. For each event type, one of the three interviewees was a physician, and the other two were nurses. We then examined the failures across events to create a common set of failures by the phases of the L&D care process (e.g., pre-hospital, triage/assessment, monitoring/laboring; Table 3).
The four final events and common failures provided a context (or stimuli) for the exploratory activities that were conducted under Aim 2. Specifically, Aim 2 sought to (1) identify if patients and family members and providers agreed about what kinds of information should be disclosed regarding each event and (2) explore how communication should occur to mitigate risk and reduce liability. The results of Study 1 were essential for conducting Study 2.
Table 1. Adverse L&D Events, Average Risk, Liability, and Teamwork Ratings (N=10)
| Survey Items | Average Risk | Average Liability | Average Teamwork |
|---|---|---|---|
|
2.10 | 4.20 | 3.30 |
|
2.60 | 4.20 | 3.60 |
|
1.60 | 3.30 | 4.80 |
|
1.40 | 4.90 | 4.60 |
|
1.50 | 4.20 | 3.90 |
|
2.20 | 2.40 | 3.70 |
|
2.00 | 2.40 | 3.90 |
|
2.65 | 2.00 | 2.80 |
|
3.00 | 4.00 | 4.90 |
|
3.90 | 2.10 | 4.80 |
|
3.20 | 1.80 | 1.80 |
|
3.70 | 1.40 | 2.00 |
|
2.00 | 2.70 | 3.00 |
|
2.60 | 2.20 | 2.70 |
|
2.20 | 1.90 | 2.60 |
|
2.30 | 2.70 | 3.20 |
|
3.60 | 1.80 | 3.10 |
|
1.90 | 1.70 | 1.60 |
| Average Rating | 2.47 | 2.77 | 3.35 |
Note: N= 10 for all average risk, liability, and teamwork scores. Risk, Liability, and Teamwork were rated on a 5-point scale where a rating of 5 was the high end of the scale, and a rating of 1 was the low end of the scale.
Table 2. Candidate and Final L&D Adverse Events (Final events appear in bold)
| Liability | |||
|---|---|---|---|
| Ratings | High | Low | |
| Risk | High | Shoulder Dystocia Birth Trauma—Injury to Neonate—Vaginal Birth |
Post-Partum Hemorrhage |
| Low | Maternal Death Intra-Partum Fetal Death due to Group B Strep Uterine Rupture |
Unplanned Admission to ICU (Mother or Baby) Unplanned Return to L&D or OR |
|
Table 3. Individual, Team, and System Failures Common Across the Four L&D Adverse Outcomes, by L&D Phase
| Common Failures | |
|---|---|
| Pre-Hospital | Clinician does not sufficiently educate patient/family about risks. |
| Clinicians do not collect adequate information on patient's history. | |
| Patient/family does not provide adequate/honest information on patient’s history. | |
| Clinician does not properly record patient’s history. | |
| Clinicians do not understand patient or patient’s family due to a language barrier. | |
| Clinician does not conduct an interview with patient privately to identify any important factors patient does not wish to be shared with other family members/father. | |
| Clinicians do not assess if patient is compliant with expectations for prenatal care. | |
| Clinician does not collect information on patient’s history with the correct people present. | |
| Individual physicians’ documentation differs within clinic. | |
| Triage / Assessment | Clinician does not sufficiently educate patient/family about risks. |
| Clinicians do not collect adequate information on patient’s history. | |
| Patient/family does not provide adequate/honest information on patient’s history. | |
| Clinician does not properly record patient’s history. | |
| Clinicians do not understand patient or patient’s family due to a language barrier. | |
| Clinician does not conduct an interview with patient privately to identify any important factors patient does not wish to be shared with other family members/father. | |
| Clinicians do not assess if patient is compliant with expectations for prenatal care. | |
| Clinician does not collect information on patient’s history with the correct people present. | |
| Patient information is documented in different places (i.e., paper and electronic). | |
| Physician and nursing documentation procedures differ. | |
| Clinician does not verbally communicate plan of care to nursing (not just electronically). | |
| Prenatal records are not available. | |
| Monitoring / Laboring | Clinician does not properly assess patient’s condition. |
| Patient’s records are inaccurate or missing. | |
| Nursing does not monitor vital signs appropriately. | |
| Clinicians do not properly monitor labor. | |
| Delivery | Clinician fails to communicate patient risk factors/situation to new clinical team members. |
| Clinicians fail to anticipate/plan for possible complications. |
Research Study 2
The goal of Study 2 was to assess how patient, family, and provider communication should occur related to the four adverse events and their common causes identified in Study 1. Specifically, we sought to identify what information should be disclosed, when it should be disclosed, and how it should be communicated in order to mitigate risk and reduce liability.
Methods
Participants
We recruited two types of participants to address Aim 2. One group comprised patients and family members, and the second group comprised clinicians.
Regarding patients and family members, individuals were recruited to participate in one of four focus groups. The purpose of each focus group was to review and discuss one of the adverse L&D events identified during Study 1. Participants were recruited from mother-baby educational classes from an 850-bed academic medical center. Because these focus groups were 2 hours long and dealt with delicate issues that might make recruitment difficult, participants were paid $200 each to participate.
Table 4 presents the demographic information on the patient and family participants in each focus group. Forty-seven participants were recruited, of whom 18 were pregnant, 28 either had experienced or had a family member that had experienced a medical mistake, and nine worked in a health care setting.
Table 4. Patient and Family Focus Group Participants
| Event | Participants | Pregnant | Experienced a Medical Mistake | Works in Health Care |
|---|---|---|---|---|
| Unplanned Return to OR or L&D for Bleeding | 10 | 5 | 7 | 1 |
| Shoulder Dystocia | 18 | 7 | 10 | 5 |
| Post-Partum Hemorrhage | 9 | 4 | 5 | 1 |
| Intra-Partum Fetal Death due to Group B Strep | 10 | 2 | 6 | 2 |
| TOTAL | 47 | 18 | 28 | 9 |
Regarding the clinician group, providers were recruited to complete a survey about what to disclose related to the common failures that can result in each of the four adverse L&D events. Thirteen clinicians—including physicians, residents, and nurses—participated in the survey. Each clinician was assigned one of the four adverse events about which to respond to the survey questions. Table 5 presents the information about the provider participants.
Patient and Family Focus Groups
Recruited patients and family members participated in focus groups that lasted 2 hours. Each focus group addressed one of the adverse event types, with all focus groups conducted using the same process. First, participants were asked to review the patient and family responsibilities identified for the specific adverse event assigned to that focus group. Following this review, the focus group leader led a discussion about disclosure and what patients and families preferred clinicians to disclose when a mistake occurred. Possible scenarios were proposed to the group to facilitate the conversation around disclosing mistakes and failures.
Table 5. Providers Surveyed About Disclosure of L&D Events
| Event | Participants | Experienced a Medical Mistake | Completed TeamSTEPPS Training |
|---|---|---|---|
| Unplanned Return to OR or L&D for Bleeding | Physicians (1) Residents (1) Nurses (1) | 3 | 1 |
| Shoulder Dystocia | Physicians (1) Nurses (2) | 1 | 0 |
| Post-Partum Hemorrhage | Physicians (2) Residents (1) Nurses (1) | 0 | 1 |
| Intra-Partum Fetal Death due to Group B Strep | Physicians (1) Nurses (2) | 1 | 0 |
| TOTAL | 13 | 5 | 2 |
Next, a survey was distributed and completed by all participants (the survey instrument is available from the authors). For each focus group, the survey presented the same series of individual, team, and system failures organized by the phases of L&D that were identified as being common to all four events. Each participant was asked to rank the failures from most important to disclose to least important to disclose by phase of L&D care for the adverse event the respective group was assigned. For example, participants in the group discussing Intra‑Partum Fetal Death due to Group B Strep ranked the failures in the context of this event, participants in the group assigned Shoulder Dystocia ranked the failures in terms of this event, and so on. We employed a ranking rather than rating process to ensure variability among events.
Clinician Survey
Using the same survey that was administered to patients and family members as part of the focus group process, we surveyed clinicians regarding their perceptions around disclosing to patients and families individual, team, and system failures that occur. As described, the survey presented the same series of individual, team, and system failures organized by the phases of L&D that were identified as being common to all four events. Each clinician was asked to rank the failures from most important to disclose to least important to disclose by L&D phase of care regarding the adverse event he or she was assigned. For example, three clinicians were surveyed regarding Intra-Partum Fetal Death due to Group B Strep, three clinicians were surveyed about Shoulder Dystocia, and so on (Table 5).
Results
Patient and Family Focus Groups
Table 6 reports the mean rating for each individual, team, and system failure ranked by the patient and family focus group participants. As described, failures were ranked from 1 (Most Important to Disclose) to 5 (Least Important to Disclose) for each phase of the L&D process. Furthermore, recall that while the failures were common across the four focus groups, the adverse obstetrical outcome that was the focus of each group was different. This approach added insight into whether type of event (high risk, high liability; high risk, low liability, and so on) moderated the type of information patients and family members wanted disclosed.
Table 6. Mean Ratings by Patient and Family Focus Group Participants for Failures by L&D Phase
| Phase | Failure | Unplanned Return | Shoulder Dystocia | Post-Partum Hem. | Intra-Partum Fetal Death |
|---|---|---|---|---|---|
| Pre-Hospital | Clinician does not educate mother/family about risks | 3.60 | 3.06 | 4.33 | 4.00 |
| Clinicians do not collect adequate information on mother's history | 4.00 | 3.56 | 3.56 | 3.50 | |
| Clinician does not properly record mother's history | 3.90 | 3.89 | 3.67 | 3.40 | |
| Clinician does not conduct appropriate prenatal tests | 3.20 | 2.22 | 2.44 | 1.80 | |
| Clinician fails to diagnose problems with mother or baby | 1.80 | 1.72 | 1.00 | 1.30 | |
| Triage/ Assessment | Clinicians do not assess if mother is compliant with expectations for prenatal care | 3.20 | 3.41 | 3.22 | 3.30 |
| Mother's information is documented in different places (i.e., paper and electronic) | 3.90 | 4.18 | 4.33 | 3.70 | |
| Physician and nursing documentation procedures differ | 3.20 | 3.53 | 3.78 | 2.70 | |
| Clinician does not verbally communicate plan of care to nursing (not just electronically) | 3.10 | 2.24 | 2.11 | 2.40 | |
| Prenatal records are not available or missing | 2.60 | 1.35 | 1.56 | 2.20 | |
| Monitoring/ Laboring | Clinician does not properly assess mother's condition | 2.90 | 1.82 | 2.44 | 3.20 |
| Mother's records are inaccurate or missing | 4.20 | 4.47 | 4.44 | 3.10 | |
| Clinicians do not follow appropriate procedures | 3.60 | 2.65 | 3.56 | 3.00 | |
| Clinicians do not properly monitor labor | 2.40 | 2.76 | 2.22 | 2.20 | |
| Clinicians do not order/administer appropriate medications | 3.30 | 2.76 | 2.33 | 2.50 | |
| Delivery | Clinician fails to communicate mother's risk factors/situation to new clinical team members | 3.00 | 2.82 | 2.44 | 2.00 |
| Clinicians fail to anticipate/plan for possible complications | 3.10 | 2.29 | 2.89 | 2.50 | |
| Clinician does not properly assess tears | 3.10 | 3.06 | 2.11 | 3.90 | |
| Nurse fails to assess mother every 15 minutes during first hour | 3.20 | 3.06 | 3.67 | 2.70 | |
| Clinician fails to instruct team members of their roles and responsibilities | 4.10 | 3.41 | 3.89 | 3.10 | |
| Post-Partum—L&D | Clinician team fails to inform the new born care team about problems with delivery | 2.10 | 2.53 | 2.11 | 2.40 |
| Clinician does not inform the patient's family about any concerns | 3.60 | 3.24 | 4.22 | 3.9 | |
| Clinical team does not educate the patient and family about "normal" behavior for the baby so they can assist in observing and reporting any abnormalities | 3.90 | 3.47 | 3.22 | 2.90 | |
| Nursing does not monitor mother's vital signs appropriately | 3.40 | 2.18 | 2.78 | 2.40 | |
| Clinician fails to communicate mother's or baby's risk factors/situations to new clinical team members | 2.90 | 3.18 | 2.67 | 2.40 |
Note: N=the number of participants ranking each failure. Unplanned Return (N=10); Shoulder Dystocia (N=18); Post‑Partum Hemorrhage (N=9); Intra-Partum Fetal Death due to GBS (N=10).
Table 7 presents the correlation among the mean rankings for each event presented in Table 6. To calculate these correlations, we computed a correlation between the participant mean rankings by phase of L&D. For example, we calculated a correlation between the rankings of failures that can occur during the Pre-Hospital phase for Shoulder Dystocia and Post-Partum Hemorrhage. These correlations provide insight as to whether the adverse event type affected how patients and family members prioritized the failures that they would want disclosed. If adverse event affected the ranking of these common failures, then the correlations would be low. If adverse event type had no effect on ranking failure, these correlations would be high. Referring to Table 7, type of event had little effect on the rank ordering of the individual, team, and system failures for patients and family members.
Clinician Survey
As reported above for the patients and family members, Table 8 reports the mean rating for each individual, team, and system failure ranked by the clinicians that were surveyed.
Similar to the analyses reported in Table 7 for patients and family members, Table 9 presents the correlation among the mean rankings for each event. To calculate these correlations, we computed a correlation between the clinician mean rankings by phase of L&D. Table 9 provides insight as to whether the adverse event type affected how clinicians prioritized the failures that they would want to disclose.
Table 7. Correlations Among the Mean Rankings for Each Event by L&D Phase
| Unplanned Return | Shoulder Dystocia | Post-Partum Hemorrhage | |
|---|---|---|---|
| Pre-Hospital | |||
| Shoulder Dystocia | 0.91 | ||
| Post-Partum Hemorrhage | 0.91 | 0.84 | |
| Intra-Partum Fetal Death due to GBS | 0.85 | 0.87 | 0.97 |
| Triage/Assessment | |||
| Shoulder Dystocia | 0.90 | ||
| Post-Partum Hemorrhage | 0.88 | 0.98 | |
| Intra-Partum Fetal Death due to GBS | 0.89 | 0.89 | 0.85 |
| Monitoring/Laboring | |||
| Shoulder Dystocia | 0.72 | ||
| Post-Partum Hemorrhage | 0.90 | 0.78 | |
| Intra-Partum Fetal Death due to GBS | 0.60 | 0.07 | 0.59 |
| Delivery | |||
| Shoulder Dystocia | 0.67 | ||
| Post-Partum Hemorrhage | 0.73 | 0.42 | |
| Intra-Partum Fetal Death due to GBS | 0.26 | 0.47 | −0.14 |
| Post-Partum L&D | |||
| Shoulder Dystocia | 0.47 | ||
| Post-Partum Hemorrhage | 0.75 | 0.55 | |
| Intra-Partum Fetal Death due to GBS | 0.54 | 0.53 | 0.94 |
Note: The N for each correlation was the number of common failures for each phase of L&D. Therefore, N=5 for all correlations.
Referring to Table 9, unlike the results from patients and family members found in Table 7, type of event had an impact on the rank ordering of the individual, team, and system failures. Event type (e.g., Shoulder Dystocia, Post-Partum Hemorrhage) mattered least during the Delivery phase, with correlations ranging from .62 to .84. Examining the other L&D phases shows quite a bit of variability in results, with the Triage/Assessment phase showing the largest range (-.58 to .72).
Table 8. Mean Ratings for Failures by L&D Phase
| Phase | Description | Unplanned Return | Shoulder Dystocia | Post-Partum Hem. | Intra-Partum Fetal Death |
|---|---|---|---|---|---|
| Pre-Hospital | Clinician does not educate mother/family about risks | 4.00 | 5.00 | 3.50 | 4.67 |
| Clinicians do not collect adequate information on mother's history | 3.70 | 2.33 | 4.00 | 3.00 | |
| Clinician does not properly record mother's history | 3.70 | 2.00 | 3.67 | 2.67 | |
| Clinician does not conduct appropriate prenatal tests | 2.70 | 3.67 | 1.67 | 1.67 | |
| Clinician fails to diagnose problems with mother or baby | 1.00 | 2.00 | 1.33 | 3.00 | |
| Triage/ Assessment | Clinicians do not assess if mother is compliant with expectations for prenatal care | 2.70 | 4.00 | 5.00 | 5.00 |
| Mother's information is documented in different places (i.e., paper and electronic) | 2.70 | 1.67 | 3.67 | 2.33 | |
| Physician and nursing documentation procedures differ | 3.00 | 4.33 | 1.67 | 3.00 | |
| Clinician does not verbally communicate plan of care to nursing (not just electronically) | 3.70 | 3.67 | 2.33 | 2.33 | |
| Prenatal records are not available or missing | 3.00 | 1.33 | 2.33 | 2.33 | |
| Monitoring/ Laboring | Clinician does not properly assess mother's condition | 2.30 | 2.67 | 1.67 | 2.00 |
| Mother's records are inaccurate or missing | 3.70 | 2.67 | 4.33 | 4.33 | |
| Clinicians do not follow appropriate procedures | 3.00 | 3.00 | 2.67 | 4.00 | |
| Clinicians do not properly monitor labor | 2.30 | 2.33 | 3.67 | 3.00 | |
| Clinicians do not order/administer appropriate medications | 3.70 | 4.33 | 2.67 | 1.67 | |
| Delivery | Clinician fails to communicate mother's risk factors/situation to new clinical team members | 2.00 | 2.67 | 3.00 | 2.00 |
| Clinicians fail to anticipate/plan for possible complications | 1.70 | 1.67 | 1.67 | 2.00 | |
| Clinician does not properly assess tears | 3.70 | 3.33 | 3.33 | 4.67 | |
| Nurse fails to assess mother every 15 minutes during first hour | 3.30 | 2.33 | 3.33 | 2.67 | |
| Clinician fails to instruct team members of their roles and responsibilities | 4.30 | 5.00 | 3.67 | 3.67 | |
| Post-Partum—L&D | Clinician team fails to inform the new born care team about problems with delivery | 2.00 | 2.33 | 3.00 | 2.00 |
| Clinician does not inform the patient's family about any concerns | 2.30 | 2.00 | 4.00 | 2.67 | |
| Clinical team does not educate the patient and family about "normal" behavior for the baby so they can assist in observing and reporting any abnormalities | 5.00 | 4.67 | 3.33 | 4.67 | |
| Nursing does not monitor mother's vital signs appropriately | 2.70 | 3.00 | 2.33 | 3.33 | |
| Clinician fails to communicate mother's or baby's risk factors/situations to new clinical team members | 3.00 | 3.00 | 2.33 | 2.33 |
Note: N=the number of participants ranking each failure. Unplanned Return (N=3); Shoulder Dystocia (N=3); Post‑Partum Hemorrhage (N=4); Intra-Partum Fetal Death due to GBS (N=3).
Comparing Clinician and Patient and Family Rankings
Finally, we compared the patient and family rankings of the individual, team, and system failures to the clinician rankings of the same failures. This analysis was conducted to determine if patients and family members agree regarding what should be disclosed and if this agreement is consistent or varies by event type. Table 10 presents the results of this analysis.
Table 9. Correlations Among the Mean Rankings for Each Event
| Unplanned Return | Shoulder Dystocia | Post-Partum Hemorrhage | |
|---|---|---|---|
| Pre-Hospital | |||
| Shoulder Dystocia | 0.41 | ||
| Post-Partum Hemorrhage | 0.89 | 0.07 | |
| Intra-Partum Fetal Death due to GBS | 0.35 | 0.49 | 0.45 |
| Triage/Assessment | |||
| Shoulder Dystocia | 0.25 | ||
| Post-Partum Hemorrhage | −0.58 | 0.00 | |
| Intra-Partum Fetal Death due to GBS | −0.46 | 0.55 | 0.72 |
| Monitoring/Laboring | |||
| Shoulder Dystocia | 0.64 | ||
| Post-Partum Hemorrhage | 0.40 | −0.28 | |
| Intra-Partum Fetal Death due to GBS | 0.21 | −0.51 | 0.66 |
| Delivery | |||
| Shoulder Dystocia | 0.83 | ||
| Post-Partum Hemorrhage | 0.84 | 0.76 | |
| Intra-Partum Fetal Death due to GBS | 0.82 | 0.64 | 0.62 |
| Post-Partum L&D | |||
| Shoulder Dystocia | 0.97 | ||
| Post-Partum Hemorrhage | 0.05 | −0.15 | |
| Intra-Partum Fetal Death due to GBS | 0.90 | 0.87 | 0.15 |
Table 10. Relation Between Patient and Family and Clinician Disclosure Rankings
| Phase | Unplanned Return to L&D | Shoulder Dystocia | Post-Partum Hemorrhage | Intra-Partum Fetal Death |
|---|---|---|---|---|
| Pre-Hospital | 0.21 | −0.08 | 0.89* | 0.62 |
| Triage/Assessment | −0.37 | 0.28 | 0.30 | 0.37 |
| Monitoring/ Laboring | 0.80* | −0.08 | 0.55 | 0.30 |
| Delivery | 0.71 | 0.86* | 0.28 | 0.96* |
| Post-Partum L&D | 0.65 | 0.39 | 0.74 | 0.12 |
| Across All Phases | 0.70* | 0.19 | 0.57* | 0.48* |
Note: * indicates significance at p<.05.
Referring to Table 10, agreement between patients and family members and clinicians regarding what failures were most important to disclose varied tremendously. The highest level of agreement was for those failures that could occur during the Delivery phase for the event Intra‑Partum Death due to Group B Strep (r=.96), while the lowest agreement was for those failures that made up the Triage/Assessment Phase for the adverse event Unplanned Return to L&D or OR (r=-.37). Across all failures, however, there was generally positive agreement among what patients and families wanted clinicians to disclose and what clinicians felt was important to disclose, with Unplanned Return to L&D or OR demonstrating the highest level of agreement (r=.70), and Shoulder Dystocia demonstrating the lowest (r=.19).
Discussion
In summary, two exploratory studies were performed to begin to understand the relation among risk and liability and the types of information patients would like clinicians to disclose. Four adverse events from L&D, that all require teamwork but vary in terms of risk and liability, were the focus of these studies. Across the two studies described here, several interesting findings emerged.
First, clinicians generally showed high agreement when assessing the degree of risk, liability, and teamwork associated with specific clinical events in L&D (Appendix A). This finding is important because procedures like Failure Mode Event Analysis (FMEA) often rely on the judgment of clinical experts regarding the characteristics of clinical events. These data seem to support that experts are in fact capable of making such judgments with fairly high levels of agreement with little or no training. Future research should test whether such judgments are also valid by comparing such judgments to independent, objective measures of these variables.
Second, patients and family members were found to agree about which failures were important to disclose, and the type of adverse event did not affect these results. Clinicians, on the other hand, showed far more variability among themselves regarding what should be disclosed; these results did appear to be affected by adverse event, although this finding may have been a result of a small sample of clinicians completing the survey. Interestingly, however, there did appear to be some agreement between clinicians and patients and family members about what failures to disclose. This finding provides some preliminary insight about the importance of disclosure, which can reduce or offset liability claims.
Limitations
Given the exploratory nature of this work, there obviously are a number of limitations. First, all the information was qualitative in nature. We used interviews, surveys, and focus groups as the core methods of our investigation. Second, our sample sizes were extremely small. Finally, our investigation was limited to one clinical domain, L&D. Collectively, these limitations make our findings potentially unreliable and difficult to replicate.
Conclusion
Despite these limitations, we do believe our work provides an important first look into the issue of disclosure and how patients and family members and clinicians perceive how best to deal with individual, team, and system failures in L&D that can lead to poor outcomes. Future empirical research needs to test the propositions we discovered here to ensure our conclusions are valid. As health care reform continues to expand and the emphasis moves from fee-for-service to quality, the role of patients and family members in the care team will also expand. Understanding disclosure and how to communicate with patients and families about sensitive care issues will be critical for enhancing patient and family engagement.
Acknowledgments
This work was supported by a grant from the Agency for Healthcare Research and Quality (HS19512).
Author Affiliations
David P. Baker, PhD, IMPAQ International, LLC; Anthony D. Slonim, MD, DrPH, Renown Health; Patrice Weiss, MD, Carilion Clinic.
Address correspondence to: David P. Baker, IMPAQ International, LLC, 10420 Little Patuxent Parkway, Columbia, MD 21044; email dbaker@impaqint.com.
References
1. Kohn L, Corrigan J, Donaldson M, eds. To Err Is Human. Washington, DC: Committee on Quality of Health Care in America, Institute of Medicine. National Academies Press; 1999. Available at https://psnet.ahrq.gov/resources/resource/1579. Accessed March 7, 2017.
2. Risser, D, Rice, M, Salisbury, M, et al. The potential for improved teamwork to reduce medical errors in the emergency department. Ann Emerg Med 1999; 34(3):373-83.
3. Mann S, Marcus R, Sachs B (2006). Lessons from the cockpit: How team training can reduce errors on L&D. Contemp Ob Gyn 2006; 1-7.
4. Agency for Healthcare Research and Quality. TeamSTEPPS Website. Rockville, MD: AHRQ. Available at https://www.ahrq.gov/teamstepps/index.html. Accessed March 7, 2017.
5. Clinton HR, Obama B. Making patient safety the center piece of liability reform. N Engl J Med 2006; 354:2205‑8.
6. Dunst CJ, Trivette CM, Hamby DW. Meta-analysis of family-centered help giving practices research. Ment Retard Dev Disabil Res Rev 2007; 13(4):370-8.
7. Denham CR. SBAR for patients. J Patient Saf 2008; 4(1):38-48.
8. Sorbero ME, Farley DO, Mattke S, et al. Outcome Measures for Effective Teamwork in Inpatient Care: Final Report. Santa Monica, CA: RAND Corporation; 2008. Available at http://www.rand.org/pubs/technical_reports/TR462.html. Accessed March 7, 2017.
Appendix