| This chapter discusses how to layer interventions to take you team's project toward excellence. It will help your team address all the failure modes in the process of VTE prevention, and how persistence, consolidation of Level 3 performance, and a variety of active surveillance techniques are the keys to success. |
The example outlines a common occurrence in implementation of hospital-associated venous thromboembolism (HA-VTE) prevention projects: although the medical center found some success from its implementation project, it hit a stumbling block. The following points demonstrate what can be done to address this kind of barrier by layering interventions and moving the effort toward excellence.
Example
Superior Medical Center has been actively working on venous thromboembolism prevention (VTE) for 18 months. Nearly a year after launching new protocol-driven VTE prevention order sets along with educational efforts, Superior Medical Center’s measures of adequate prophylaxis improved from 60 percent to 80 percent on audits, and SCIP VTE measures are routinely around 95 percent—yet the incidence of hospital-associated VTE has not really improved. How should the VTE prevention team respond to the situation? |
Reviewing the Basics—Order Set Design and Implementation
If this happens, a productive and appropriate response would be to reassess efforts and make sure the foundation for improvement is in place—and then to layer on interventions in order to gradually achieve near-perfect prophylaxis.
Whether orders are on paper or in computerized physician order entries (CPOEs), every attempt should be made to integrate the VTE prophylaxis protocol into the processes for admission and for transfer from one hospital unit to another. This will require that the VTE prevention orders be tightly linked to all appropriate admission, transfer, and perioperative order sets. Better yet, the VTE prevention orders can be integrated into these larger order sets as a standard component, with all nonstandardized reference to VTE prophylaxis removed. Audits of order set use, dialogue with physicians, and direct observation can be carried out and modifications made until the great majority of patients are reliably assessed for VTE risk and assigned risk-appropriate prophylaxis on admission and transfer.
Beyond the Basics—Addressing Failure Modes and Layering Interventions
A variety of failure modes commonly occur in quality improvement interventions. Table 7.1 outlines the failure modes introduced in Chapter 2, along with strategies and solutions to address each. The first four failure modes are addressed by optimizing order set design and integration, reaching Level 3 on the Hierarchy of Reliability. Improvement teams can then start moving beyond the basics to address the other failure modes in the table.
Table 7.1: Common Failure Modes in Providing Optimal VTE Prophylaxis and Strategies and Solutions To Address Them
| # | Failure Mode | Strategies and Solutions |
|---|---|---|
| 1 | No standardized protocols or order sets for VTE prevention exist. | Standardize VTE prevention and codify in an institutional protocol. Embed VTE protocol guidance in admission, transfer, and perioperative order sets. |
| 2 | Order sets/prompts that reference VTE prevention are in place but provide inadequate guidance. | Provide guidance for VTE risk assessment, bleeding, and prophylaxis choices for each combination of VTE and bleeding risk factors. Provide explicit operational definitions (e.g., cutoff for low platelets, definition of “ambulatory”). |
| 3 | Order sets with guidance are in place, but the order set is bypassed or not used. | Hard stops to make completion of order sets mandatory. Algorithmic design that allows ordering of prophylaxis only after VTE risk and bleeding risk assessments are complete. Active surveillance to detect those bypassing order sets. |
| 4 | Order sets with guidance are in place and used, but used incorrectly. | Education. Refinement of order sets to make them less ambiguous. Active surveillance to detect improper use of the order set. |
| 5 | Patient gets placed on right prophylaxis, but VTE/bleeding risk changes and adjustment is not made. | Education. Integrate VTE prophylaxis assessment into checklists or care pathways, especially in critical care units and elective surgery patients. Audit and feedback. E-alerts or human alerts. Measure-vention. |
| 6 | Prophylaxis gets missed/changed on transfer/perioperative setting. | Hard stop for VTE and bleeding risk assessment and VTE prevention orders postoperatively and with every transfer to a different level of care. |
| 7 | Correct prophylaxis is ordered but not administered, or patient refuses. | Education programs for nurses and patients. Engage patients in the process. Audit and feedback. Measure-vention. |
| 8 | Patient is not mobilized optimally. | Progressive activity and mobility programs. Measure-vention that incorporates mobility. Flow sheets that juxtapose activity orders with actual performance. Education. |
| 9 | Preventable risk factors (central line) are not optimally managed. | Central line/peripherally inserted venous catheter programs to minimize excessive use of central lines and ensure proper insertion and maintenance. Use smallest caliber lines possible. |
| 10 | Prophylaxis is stopped at discharge even though the patient has indications for extended duration prophylaxis. | Define populations that require extended duration prophylaxis and embed in clinical pathways and discharge checklists. Case management or discharge pharmacy should ensure patient can obtain extended duration prophylactic agent prior to discharge. |
Intervention strategies can be either passive or active. Passive interventions, such as educational sessions and posters, can be useful, but they are generally not as effective as active interventions that provide clinical decision support (CDS), reminders, and correction of lapses in care.1-7 Active surveillance techniques find ways to detect potential failures in the process and intervene to correct the lapse in care on a regular basis. Active interventions are described in more detail below.
Use Checklists, Prompts, and Care Pathways That Reinforce VTE Prevention Order Sets
Reminders for VTE prophylaxis can be integrated into history and physical forms, critical care rounding tools, and a variety of other instruments. A simple checklist can spur meaningful improvement in a powerful way, and checklists that incorporate VTE prophylaxis can be particularly useful in perioperative and critical care settings.4,6,8 These strategies are most effective when used as a redundant mechanism (rather than as primary strategies) to leverage the VTE prevention order set.
Many institutions integrate VTE prophylaxis into care pathways, particularly for major orthopedic surgery, neurosurgery, and other selected surgical procedures.5-7 Populations that may benefit from extended duration prophylaxis (e.g., major orthopedic surgery patients, patients with abdominal/pelvic surgery with cancer) should have VTE prevention measures in their care pathway or discharge checklist. Aligning the checklist and care pathways with the VTE prevention protocol embedded in the order sets is crucial to avoid confusion and mixed messages.
Consider Alerts To Improve Appropriate Orders for Prophylaxis
The benefits of electronic alerts (e-alerts) to increase thromboprophylaxis and reduce VTE rates among hospitalized patients have been demonstrated in clinical trials.9,10 Almost 2,500 high-risk patients identified by data available in the electronic health record who were not receiving prophylaxis were assigned to an intervention or control group. In the intervention group, the treating physician received an unsolicited e-alert, resulting in improved prophylaxis rates and fewer VTE events at 90 days.9 A study using human alerts, rather than e-alerts, provided similar findings, demonstrating that electronic health records are not required to use this strategy.11 These findings suggest that alerts are useful—but they should complement other strategies to be most effective.
Active surveillance alerts have up to three steps:
- A screen or filter that identifies a current potential lapse in care.
- A rapid triage step in which the potential lapse in care is confirmed or denied.
- An intervention that entails some form of notification to the ordering provider.
There was no triage step in the studies discussed above, and the screening step triggered an alert without any further adjudication. When this strategy is used, having the screening step err on the side of being specific, rather than sensitive, for those at VTE risk can reduce false alerts and alert fatigue.
Use Audit and Feedback To Assess Performance and Provide Targeted Education
Traditional audit and feedback, accomplished by giving periodic reports to provider groups on their performance on VTE prophylaxis, has also demonstrated some success.1,2,12 Posting results and making them public can be an effective method, but it needs to be approached with sensitivity to the political climate and, ideally, with the permission of the physicians involved. Audit and feedback can also help target groups that might benefit from educational detailing.
Address Reliability of Prophylaxis Administration, Adherence, and Prescribing
Clinical decision support, order sets, and the like are strategies to increase the rates of appropriate prescribing, but the reliability of administering the ordered prophylaxis is often suboptimal, particularly for mechanical prophylaxis.13-15 One prospective study of postoperative patients with deep vein thrombosis (DVT) risk factors and mechanical prophylaxis orders found that patients on routine nursing units had properly functioning intermittent pneumatic compression (IPC) devices during 48 percent of the visits, while ICU patients had them 78 percent of the time.13 Another observational study had similar findings, with errors in mechanical prophylaxis being present in 44 percent of the observations of patients with orders for IPC alone, and 56 percent with errors when combination mechanical and anticoagulation prophylaxis were ordered. Errors in misapplication of the IPC devices and errors of omission both played roles.15 Sites enrolled in Society of Hospital Medicine collaborative VTE improvement programs frequently reported similar findings.16,17
The difficulty in attaining high rates of mechanical prophylaxis adherence has two major implications. First, even though current guidelines make this an acceptable choice for selected subsets of surgical patients, health care providers may want to reconsider mechanical prophylaxis as a primary means of VTE prevention in those without contraindications to anticoagulation. Second, improvement teams should find methods to monitor mechanical prophylaxis administration and improve the reliability of administration.
Targeted education of nurses can be modestly effective in improving mechanical prophylaxis adherence but should not be relied on as a sole strategy. Patient engagement and education programs can reduce patient refusal of prophylactic measures. Society of Hospital Medicine collaborative VTE improvement sites have had success when coupling education with monitoring and intervention programs.16,17
Active surveillance techniques can be used to monitor and improve on reliable administration of mechanical prophylaxis. One strategy is to create a report that lists each patient on a given unit, juxtaposing the order for mechanical prophylaxis with the nursing documentation that the mechanical prophylaxis is on and in place. Some sites generate a roster of patients with orders for mechanical prophylaxis and then directly observe whether the mechanical prophylaxis is being administered on a regular basis.
Recently, portable, battery-powered intermittent pneumatic compression devices (IPCDs) have been designed to increase compliance in the hospital by offering untethered use. This allows patients more mobility and the ability to maintain use while traveling to other areas of the hospital for testing. (A small meter embedded in the device monitors the time the device is on and in place.)
Pharmacologic prophylaxis represents another opportunity to address failures in the VTE prevention process.18-21 Figure 7.1 shows the result of an electronic audit of pharmacologic prophylaxis delivery. In a 7-month period, 9.3 to 11.7 percent of ordered doses were not administered. More than 60 percent of the time, the reason given for not administering the dose was patient or family refusal.
Figure 7.1: Percentage of Ordered Subcutaneous Pharmacologic Prophylaxis Doses That Were Not Administered to Adult Inpatients
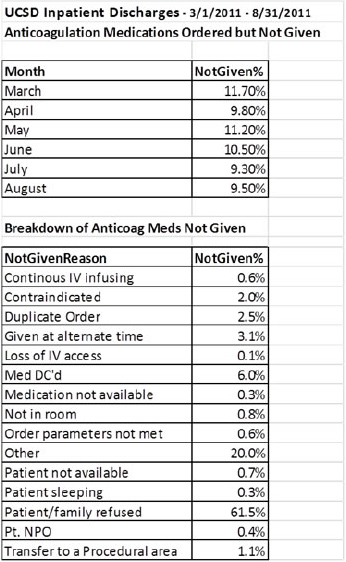
The chart shows both the doses not administered and the reason the dose was not given as recorded in the medication administration record.
Published studies have similar findings, with 10 to 15 percent of ordered doses not administered.18,21 The studies found substantial heterogeneity in nonadministration among patients, floors, and floor types, and a relatively small proportion of patients who missed multiple doses represented the majority of administration failures.21 This represents an opportunity to focus improvement efforts efficiently.
Address Central Venous Catheter-Related VTE/Upper Extremity DVT
Upper extremity DVT (UE DVT) events constitute 30 to 40 percent of hospital-associated DVT. Central venous catheters (CVCs) are the predominant cause of UE DVT, and the overall incidence of UE DVT has increased coincident with the increasing use of peripherally inserted central catheters (PICCs).22-25 The incidence of symptomatic DVT following CVC placement is 2 to 6 percent, with an 11 to 19 percent risk of asymptomatic DVT.22 Larger catheter size, infection, and improper placement are all strongly associated with DVT risk. Comorbidities such as cancer, diabetes, and trauma increase DVT risk, as do infusions of chemotherapy, antibiotics, or TPN through the CVC.22,25
The cost and length of stay attributable to PICC-associated DVT has been estimated at $15,973 and 4.6 days, respectively.23 PE associated with UE DVT has been reported as high as 12.4 percent.22 The annualized recurrence rates, while lower than lower extremity DVT, are still substantial at 2.3 to 4.7 percent.22 Removal of the CVC and several months of therapeutic anticoagulation are generally recommended, with all the potential for bleeding risk that entails.18
Appropriate VTE prophylaxis for comorbidities may be modestly beneficial in preventing UE DVT, but American College of Chest Physicians (ACCP) guidelines recommend against routine use of anticoagulant prophylaxis solely on the basis of an indwelling CVC. Significant reductions in UE DVT rates are feasible, however, by other methods. One tertiary trauma center reported a reduction in PICC-associated DVT from 3.0 percent to 1.9 percent; interventions included interdisciplinary consensus on the need for each PICC, early PICC removal, assurance of proper placement, use of a PICC with the smallest number of lumens required, and a change to smaller diameter PICCs.23 These changes are inexpensive, save money, and reduce morbidity. Improvement teams may wish to examine their practices around PICC and other CVC placements and make similar changes when needed.
Improve Mobility/Activity
Reduced mobility is common in the inpatient setting, and immobility is a well-established and powerful risk factor for HA-VTE. In fact, relative immobility in the hospital is a risk factor for delirium, decubiti, ileus, deconditioning, bone loss, and prolonged loss of cognitive and physical function.26-30 Early ambulation programs have improved outcomes after surgeries, including major orthopedic surgery.31-33 Early ambulation and progressive mobility protocols seem to hold great potential as a nonpharmacologic method to improve outcomes, including VTE, across a number of inpatient populations without the potential consequences of adverse drug effects.34-38
There are many barriers to mobility during hospitalization. Patient-related factors, such as severity of illness, dementia, pain, and weakness make mobility difficult. Concern about falling, inadequate staffing, and attitudes about the priority of mobility during hospitalization can also act as barriers.26 Many barriers to mobility are modifiable, however, including unnecessary physician orders for bed rest, oversedation, and overuse of Foley catheters, central catheters, and restraints.
Many programs are beginning to overcome these barriers and are finding that progressive mobility programs are feasible even in mechanically ventilated and critically ill patients.34-38 Strategies to improve mobility include:
- Using mobility and activity order sets that make progressive mobility the default rather than "activity ad lib" or bed rest.
- Reducing sedatives, restraints, and inappropriate Foley catheters.
- Exploring the use of nonnarcotic measures for pain control.
- Using sequential compression devices only when appropriate to do so.
- Standardizing mobility/ambulation orders and aligning them with physical therapy and nursing terminology.
- Juxtaposing the expectations for mobility and activity with the mobility actually achieved in flow sheets.
- Implementing patient and nursing education and engagement programs.
VTE improvement teams might wish to survey their institution for services that already have aggressive mobility programs and attempt to spread them to a broader segment of patients.
Achieving Measure-vention: Reaching Level 5 on the Hierarchy of Reliability
Under the stoplight method of measurement (Chapter 6), the medication administration record or an automated alert identifies the active VTE prophylaxis orders for each patient on the ward as "green" (therapeutic or prophylactic anticoagulant ordered), "yellow" (mechanical prophylaxis is the sole method of prophylaxis ordered), or "red" (no prophylaxis ordered). Lab contraindications to anticoagulant, the declared VTE risk level from the ordering physician, activity status from the Braden scale, and documentation of sequential compression device (SCD) application can be pulled into the report, thereby capturing prophylaxis patterns and a number of other factors that influence prophylaxis choices.
Measure-vention occurs when this form of measurement is coupled with intervention to correct identified lapses in care on a daily basis. When measure-vention happens, very rapid improvements in VTE prophylaxis can result.16,39,40
Measure-vention is an active surveillance strategy that addresses multiple failure modes simultaneously. The measurement portion of measure-vention can start very early in the improvement process, while the intervention coupled with this measurement is best deployed after the protocol-driven order set is well integrated into the admission and transfer process. In other words, Level 3 on the Hierarchy of Reliability should be in place before embarking on measure-vention, which can elevate performance to Level 5. Figure 7.2 offers a graphic representation of the measure-vention process.
Measure-vention, the concurrent identification and relay of quality outliers to the frontline care team, simultaneously represents an intervention and a measurement system. In the Hierarchy of Reliability, measure-vention is a Level 5 quality improvement strategy.
Figure 7.2: The Measure-vention Process
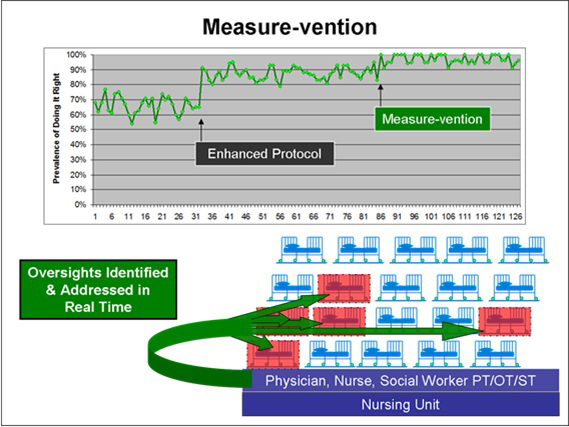
Figure 7.3 shows an excerpt from a measure-vention tool that illustrates the screening and triage process. A member of the health care team on the unit would receive this report on a daily basis or access it on demand. Generally, this daily screening is performed by a nurse, although in some institutions a pharmacist or other provider performs measure-vention.
Figure 7.3: Excerpt From Automated Measure-vention Screening Tool (Enhanced Stoplight Method)
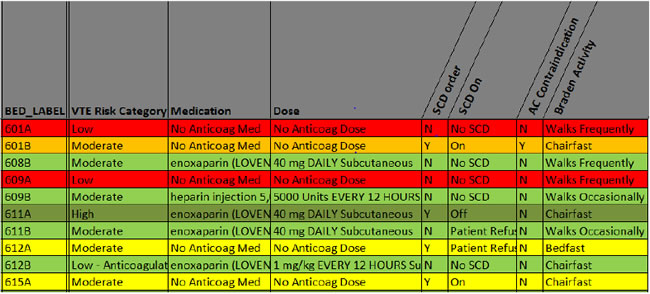
Potential under-prophylaxis is identified by patients on no prophylaxis (in the red). In this case, both “red” patients were declared low risk by their physician and have documentation of walking frequently; by protocol, no prophylaxis is required.
Patients on mechanical prophylaxis may also be subject to scrutiny. Mechanical prophylaxis is often not an acceptable form of prophylaxis in the absence of contraindications to anticoagulants at a prophylactic level. The patient in 601B has SCDs and a documented lab contraindication to anticoagulant. The color coding for this situation (orange) makes this acceptable combination known to the nurse at a glance. The patients in 612A and 612B, meanwhile, both have mechanical prophylaxis, a declared moderate VTE risk level, impaired mobility, and no contraindication for anticoagulant captured in the laboratory. These cases may represent under-prophylaxis and an opportunity for intervention if the nurse does not pick up any other obvious contraindication to anticoagulant during triage (such as scheduled surgery that day, active bleeding, or epidural insertion or removal). In addition, patient refusal of SCDs will need to be confirmed after education is provided to the patient. Finally, potential over-prophylaxis is identified by "green" patients on anticoagulant who are ambulating actively outside their rooms.
This example demonstrates how automated reports can pull together much of the information required to screen for potential care deficiencies, and assist in rapid triage, to confirm or deny a potential lapse in care.
Piloting measure-vention on one or two units is a great way to reduce false alarms and work out any bugs in the process. When measure-vention is done well, the number of cases requiring intervention goes down very rapidly and rates of adequate prophylaxis improve in just a few days. Figure 7.4 shows the results reported at Emory’s hospitals, which have been replicated by others in collaborative QI efforts.16,39,40
Figure 7.4: Piloting Measure-vention at Emory’s Hospitals
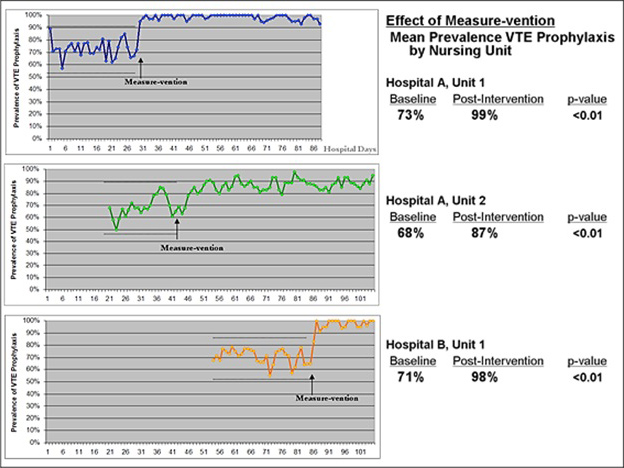
Figure courtesy of Dr. Jason Stein.
In a performance improvement pilot in Emory’s hospitals, measure-vention was initiated on three different nursing units over 100 days. All three units were at Level 3 on the Hierarchy of Reliability, with high-quality VTE order sets in place and adequate VTE prophylaxis rates hovering around 70 percent. In this staggered time series, measure-vention quickly resulted in a statistically significant increase in the prevalence of adequate VTE prophylaxis in all three units.
Taking Measure-vention to the Next Level
In an ideal world, every patient would have bleeding and VTE risk factors reassessed each day. While this is too labor intensive and difficult to perform reliably in most hospitals, some are now starting to leverage the electronic health record to automatically pull important VTE risk factors (such as cancer diagnoses and mobility) and bleeding risk factors (such as high INR and low platelets) on at least a daily basis. This dynamic process is beyond the reach of most hospitals, but pioneers are beginning to show that this natural extension of measure-vention techniques may be feasible.



