Overview of the Task Order for SATIS-PHI/CRC Development
CNA developed this toolkit as part of an AHRQ ACTION task order involving a population-based case study. It was designed to assess how easily a health system redesign intervention shown to improve colorectal cancer (CRC) screening rates and rates of diagnostic followup for positive screens in one clinical setting could be transferred to another setting. The goal was to achieve similar rate improvements.
The components of the SATIS-PHI/CRC intervention are based on prior studies conducted by project staff at Thomas Jefferson University (TJU) (Myers, 2007; Myers, 2001; Myers, 2004), which demonstrated the following:
- A targeted outreach intervention to patients in a large urban academic practice improved CRC screening rates.
- A feedback intervention to providers in practices affiliated with a large, for-profit managed care organization improved diagnostic followup for positive screens.
This project built on the cited studies and examined both:
- The process of implementing the SATIS-PHI/CRC intervention in the Lehigh Valley Physician-Hospital Organization (LVPHO) network of community-based practices (how and how well the intervention could be implemented).
- The outcome of the intervention (the degree to which it could achieve similar rate improvements for both CRC screening and followup in this different setting).
Description of the SATIS-PHI/CRC Intervention
The SATIS-PHI/CRC intervention is designed to:
- Influence the behavior of primary care providers and their patients regarding CRC screening and followup through targeted communications.
- Facilitate the screening and followup process through improved eligibility identification and screening tracking systems.
The SATIS-PHI/CRC intervention has the following features:
- Uses system-level redesign.It is a population-based, system-level redesign of the way CRC screening and followup are conducted in a network of primary care practices. It is intended to assist these practices to better provide guideline-based preventive health care to their age-appropriate patients (50 through 79 years old) who are at average risk for CRC.
- Provides system support to CRC screening. It assists practices in providing population-based CRC screening that follows recommendations and guidelines jointly issued in 2008 by the American Cancer Society, U.S. Multi-Society Task Force on Colorectal Cancer, and American College of Radiology (Levin, et al., 2008) and in 2008 by the U.S. Preventive Services Task Force (USPSTF, 2008).
- Provides a mechanism for identifying patients in need of CRC screening. It provides a mechanism for identifying patients who are eligible for but not up to date in their CRC screening, contacting such patients on behalf of their physician's practice to encourage and facilitate recommended screening, tracking screening results, and facilitating patient notification and appropriate followup through feedback to providers.
- Provides a mechanism for CRC screening. It also provides a facilitating mechanism for patients to undergo screening. Although the Multi-Society and USPSTF guidelines identify a range of acceptable screening modalities, in an effort to avoid possibly confusing patients with too many choices, SATIS-PHI/CRC limits the choice to only two: (1) a less invasive modality patients can perform themselves at home (stool test) and (2) a more invasive modality requiring a physician-performed procedure (colonoscopy).
- Educates clinicians and practice staff. Using academic detailing and performance feedback forms, it seeks to educate clinical providers and other staff in participating practices about recommended CRC screening and followup procedures.
- Educates patients. Using mailed information, it seeks to educate targeted patients of participating practices about the importance of and need for CRC screening and about the various types of recommended screening modalities.
Key Features of SATIS-PHI/CRC:
- Uses system-level redesign.
- Provides system support to CRC screening.
- Provides a mechanism for identifying patients in need of CRC screening.
- Provides a mechanism for CRC screening.
- Educates clinicians and practice staff.
- Educates patients.
The two educational components (educating clinicians and staff and educating patients) are aimed at informing and changing the behavior of clinicians and practice staff and their patients. Educating both the provider and patient about the importance of CRC screening has been shown to be effective in increasing screening in patients (Levy, 2007; Geller, 2008; Zapka, 2008). The practice/clinician component consists of academic detailing and performance feedback, whereas the patient component consists of mailed information about CRC and screening.
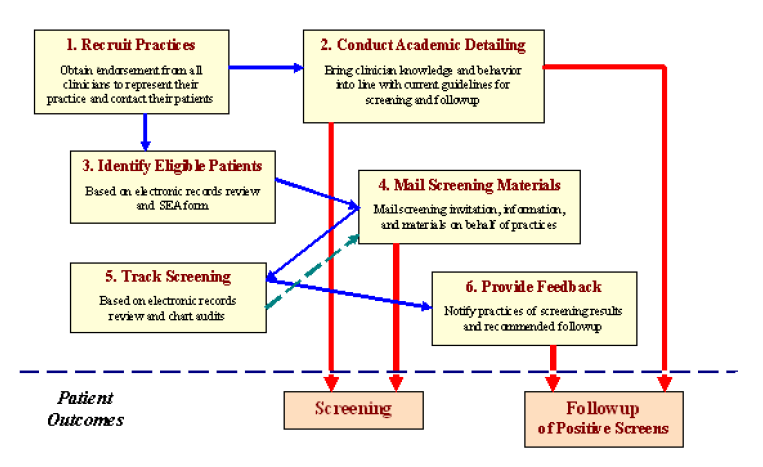
Figure 1 presents the conceptual framework for the six steps of SATIS-PHI/CRC that are conducted by the central entity:
- Step 1 brings primary care practices and their patients into the intervention.
- Step 2 seeks to influence the screening knowledge and behavior of providers within those practices and, along with Step 6, to influence followup knowledge and behavior. By educating and influencing providers, Step 2 also seeks to ensure that providers will influence the screening behavior of their patients.
- Step 4 more directly seeks to influence patient screening. The remaining steps facilitate the process.
- Step 3 identifies patients who are eligible (based on prevailing screening guidelines) to receive the Step 4 screening materials.
- Step 5 tracks patient screening and results. Those with no evidence of being screened receive a reminder Step 4 mailing, whereas the practices of those patients with evidence of screening are notified of screening results and receive feedback regarding recommended followup through Step 6.
Figure 1. Framework for S ATIS-PHI/CRC
Description of the Intervention Setting, Transferability to Other Settings, and the Central Entity
Intervention Setting
The health system setting for this project was the LVPHO formed in 1993 by the Lehigh Valley Health Network (LVHN) and the Greater Lehigh Valley Independent Practice Association (GLVIPA). The PHO, which offers a preferred provider organization health insurance plan, had an interest in value-based health care and sees preventive care, including CRC screening, as a means to that end. The LVPHO, in conjunction with the Eastern Pennsylvania Inquiry Collaborative Network (EPICNet, a practice-based research network [PBRN] of primary care practices affiliated with LVHN), was the central entity for this implementation of SATIS-PHI/CRC.
Transferability to Other Systems/Settings
Based on assessment findings and lessons learned, CNA believes that the SATIS-PHI/CRC can be a transferable intervention that can improve CRC screening and followup. It is most transferable to health care system settings with a central entity that:
- Is motivated to take the lead in organizing and implementing the effort.
- Has easy access to up-to-date and reasonably complete electronic records.
- Understands and accepts the time and resource commitment needed to undertake the intervention.
- Has experience with large, targeted, population-based mailings to patients (either by conducting such mailings themselves or outsourcing them to reliable contractors).
- Has strong relationships with its affiliated primary care practices.
Environmental conditions most supportive of successful implementation include (1) a sufficient number of willing colonoscopy providers serving the medical service areas participating in the intervention to accommodate any increased demand for colonoscopies resulting from the intervention and (2) no other competing population-based initiatives occurring in the service area or at the participating practices that could detract from the support and attention needed to implement the SATIS-PHI/CRC intervention.
CNA's experience with SATIS-PHI/CRC also demonstrates that this intervention can be successfully implemented in a wide range of practices. These include practices that are more closely and less closely affiliated with the central entity and those that have and do not have fully functional electronic medical record (EMR) systems. However, the central entity would need access to sufficient other electronic records (in particular claims or other evidence of medical services provided to patents) for practices without fully functional EMR systems. Participating practices can also enhance implementation if they are dedicated to population-based preventive health in general and have strong leaders who support the SATIS-PHI/CRC intervention effort. A clinical champion who supports the effort can also help.
Central Entity
The intervention is intended to be conducted by a central entity, such as a health care delivery system or insurer, affiliated with a network of primary care practices on behalf of and in conjunction with those practices. The central entity completes the six implementation steps highlighted in Figure 1 and described in detail in Section 3. In addition, the central entity can implement an optional assessment step that is described in Section 3.
Intervention and Assessment Steps for the Central Entity:
- Recruit practices.
- Conduct academic detailing.
- Identify eligible patients.
- Mail screening materials.
- Track screening.
- Provide feedback.
- Conduct assessment (optional).
To conduct the SATIS-PHI/CRC intervention, the central entity needs to meet the following eight requirements:
- Determine patient eligibility. It must be able to centrally and electronically determine likely eligibility based on the guideline recommendations.
- Contact patients. It must be able to contact patients on behalf of the practices to confirm their eligibility, invite them to be screened, and remind them if they have not responded after a period of time.
- Track patient responses and screening. It must be able to track patient response to the invitation and to screening results.
- Contact clinicians with screening results. It must be able to provide results to practice clinicians and remind them of appropriate recommended followup for positive screening findings.
- Obtain funding. It must be willing to fund or find funding for the intervention.i
- Recruit stool test kit suppliers. It must identify one or more suppliers of stool test kits and one or more clinical laboratories to process them (the processing labs may also be the suppliers in many cases).
- Establish relationship with a clinical lab. It must have or develop a business associate relationship with the clinical labs or use a lab it operates to allow the lab to report screenings and results to the central entity.
- Work with colonoscopy providers. It should be able to notify colonoscopy providers (identified by the participating practices) that this screening program will take place and when it will occur, and that they should expect a potential increase in requests for screenings. This last condition is recommended rather than strictly required.
Requirements for the Central Entity:
- Determine patient eligibility.
- Contact patients.
- Track patient responses and screening.
- Contact clinicians with screening results.
- Obtain funding.
- Recruit stool test kit suppliers.
- Establish relationship with a clinical lab.
- Work with colonoscopy providers.
Intervention Findings
CNA successfully implemented the intervention in the LVPHO/EPICNet setting and generally improved CRC screening rates. However, a number of factors hindered implementation and likely caused lower than expected screening rates. These included:
- Lack of implementation infrastructure. The LVPHO/EPICNet central entity lacked some elements of the ideal implementation infrastructure (in particular, electronic records systems set up for public health population-based patient outreach programs). It also lacked experience implementing a program of this size based at the central entity rather than the practices.
- Time of economic uncertainty. CNA implemented the intervention during a time of economic uncertainty and limitations, resulting in fewer staff resources available for implementation at both the central entity and the practices. They also had to change the intervention protocol to accommodate a decision by the stool test kit supplier to restrict the number of free kits it would make available. Thus, the kit could only be sent directly to a small subsample of patients; most patients had to request a kit by mailing a request card.
- Primary care transformations. The implementation timeframe was also during a time of change among primary care practices in the Lehigh Valley that affected both their electronic medical record systems and their ability to focus on the SATIS-PHI/CRC intervention.
- Implementation delays. Implementation delays shortened the period available for observing screening and followup.
- Colonoscopy provider shortages. Shortages of colonoscopy providers in the Lehigh Valley to accommodate an increase in demand for screening likely depressed observed screening and followup rates during the shortened observation period.
These factors affected CNA's ability to fully eliminate ineligible patients from the rate denominators and to fully identify completed screenings (especially colonoscopies) for the rate numerators, leading to low observed screening rates.
Despite these implementation shortcomings, CNA found significantly greater odds of patients of intervention practices than of control practices being screened during the observation period. This finding persisted even after controlling for age, gender, and various practice attributes, including the completeness of the tracking data available. Factors increasing the odds of being screened included receiving the stool test kit directly rather than having to request it and having commercial insurance.
The observed screening rate was substantially lower than in the previous study on which CNA modeled the patient outreach elements of SATIS-PHI/CRC. But when CNA more closely approximated the research conditions of that earlier study, the rate more closely approximated that study's rate. In particular, when stool test kits were sent directly to patients rather than request cards and when CNA included only patients responding to the baseline screening eligibility assessment (SEA) survey in the analysis, rates were more comparable.
Evidence showed that 786 patients were screened: 682 (8.6 percent) from intervention practices and 104 (3.9 percent, or 4.7 percent with an adjusted denominator needed for comparison purposes) from control practices. Of those 786 screens, 363 were by stool test (almost all of the others were by colonoscopy). Only 8 were positive (abnormal); CNA could not ascertain the results of an additional 18.
CNA tracked the followup experience of the 26 patients with positive or inconclusive screens for evidence of complete diagnostic examinations. Their small number precluded any meaningful assessment of the intervention's effectiveness in improving followup rates. A comparison of the pre- and postintervention survey of intervention practices suggests that the academic detailing element of SATIS-PHI/CRC was somewhat effective in educating providers about current CRC screening guidelines.
Overall, implementation of the SATIS-PHI/CRC intervention in the LVPHO/EPICNet setting demonstrates that CNA (1) successfully implemented the SATIS-PHI/CRC intervention in a setting that differed from those that prevailed in studies on which CNA based development of SATIS-PHI/CRC and (2) achieved comparable effectiveness in improving the odds of screening among guideline-eligible patients who were not up to date in their screening. Furthermore, CNA extracted a set of "lessons learned" from their implementation experience that could help others to successfully implement SATIS-PHI/CRC in their settings and to achieve comparable outcomes. These lessons learned are presented as tips in the toolkit.
i This funding is minimal considering the potential public health benefit to be derived. It is primarily for conducting electronic reviews of records, mailing material to patients, and providing stool test kits for those patients preferring to be screened by stool testing.



