| This chapter provides a roadmap and tools to start the team off on the right foot. As the team makes progress on each step, the next steps will tend to unfold in a logical progression. |
Quality improvement (QI) teams must be set up for success and can only proceed with the support of their institution and an understanding of the local environment. Teams must anticipate milestones, set goals, and use a framework for improvement.
This guide provides QI teams with information they need to progress through the framework for improvement identified in Figure 1.1. This framework serves as an outline for the guide. Users of this guide will have the best chance of success if they follow the Essential First Steps (below) before embarking on the framework.
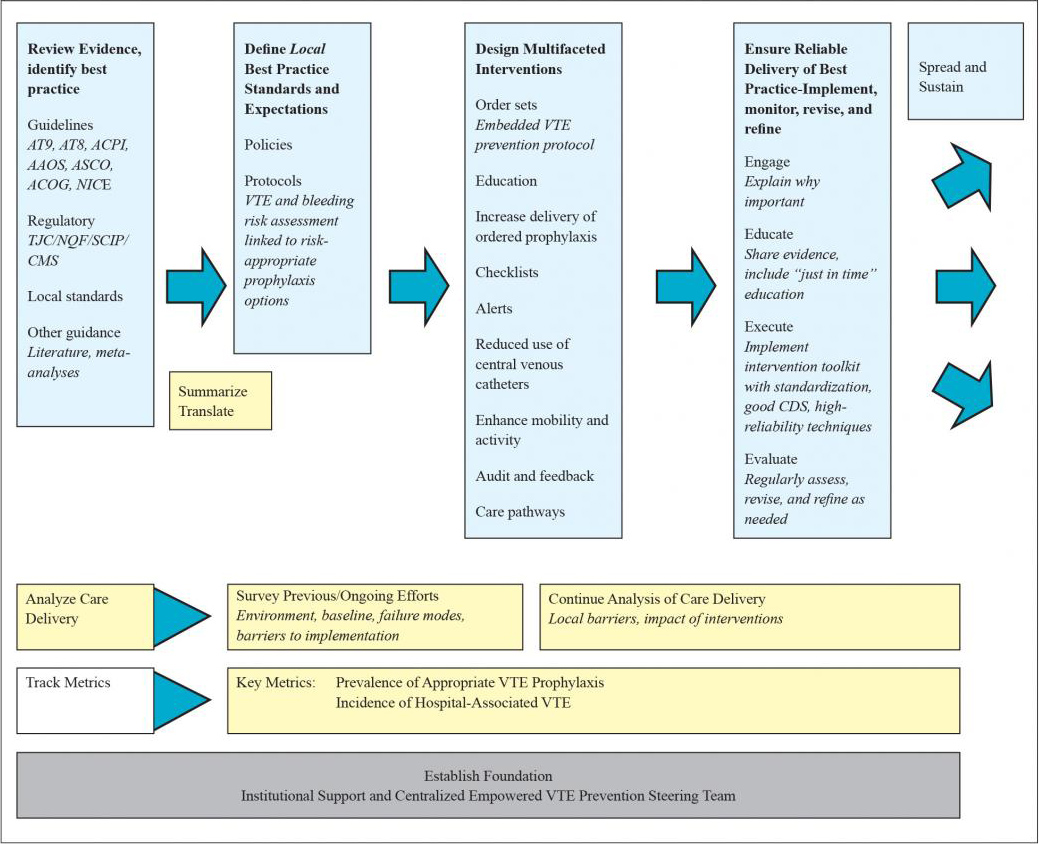
Figure 1.1 depicts a framework for formulating a protocol and deploying multiple interventions designed to reinforce the guidance from the venous thromboembolism (VTE) prevention protocol.
Figure 1.1: Framework for Improving VTE Prevention
Essential First Steps
Step 1: Ensure Support From the Institution
The time, energy, and expertise of a clinician leader are necessary to drive improvement. Alone, however, they will not be enough. Sponsorship and support from the medical institution, and specifically from key leaders, are essential. True institutional support will be reflected in prioritization of the effort, including providing resources for good measurement of progress and by the institutional will to standardize the process even in the face of physician resistance.
Real support confers on the improvement team the authority and resources needed to design and manage change. The single most effective way to attract this support is by aligning the goals of the quality improvement effort with the strategic goals of the organization, and by understanding how VTE prevention efforts fit into the larger QI ecosystem.
VTE prevention is just one priority among many for busy clinicians and QI leaders, so it is helpful to make hospital leadership aware of how an effective VTE prevention program aligns with its many other goals for medical care, performance reporting, customer service, patient safety, and cost containment.
A number of forces may fuel leadership interest in the project, including public reporting of hospital performance (e.g., The Joint Commission [TJC] and National Quality Forum [NQF] measures), Partnership for Patients initiatives, cost savings from more efficient care, risk aversion, favorable payments for better care (e.g., pay for performance), nursing and medical staff retention (e.g., Magnet Recognition Program®), related projects (e.g., Surgical Care Improvement Project), and even quality for quality’s sake. Furthermore, the Centers for Medicare & Medicaid Services no longer reimburses for the incremental costs of DVT and pulmonary embolism (PE) related to some surgeries (including total knee replacement and total hip replacement), and is considering expanding that list. VTE prevention efforts can also be synergistic with efforts to increase patient activity, reduce central venous catheter complications, and meet meaningful use criteria for electronic health records.
An argument to leadership can also be made in terms of VTE incidence and costs. Queries from the University of California system and the University Healthcare Consortium provide estimates that are consistently 1 percent or more of admissions resulting in an HA-VTE. This means that a medical center with 10,000 adult discharges per year could expect to have 100 events of HA-VTE, many of them potentially preventable. The rate of hospital-associated VTE likely remains grossly underestimated, however, as reporting does not include patients readmitted to other hospitals, undiagnosed but clinically important VTE, and VTE that is treated in skilled nursing facilities and outpatient environments.
Each hospital-associated DVT event represents an incremental cost of $7,700 to $10,800, while each hospital-associated PE event represents $9,500 to $16,600 in additional costs. Acute HA-VTE in cancer patients bears an even higher cost, estimated at more than $20,000 per episode.26 As high as this cost is, it does not reflect the longer term costs to society and the patient of recurrent VTE, post-thrombotic syndrome, and pulmonary hypertension.
|
A venous thromboembolism prevention protocol incorporates VTE and bleeding risk assessment tools and risk-appropriate prophylactic options. Relying on order sets alone will not reach desired levels of appropriate VTE prophylaxis. Analyzing care delivery, assessing and addressing barriers, and ongoing measurement and monitoring are also essential. The QI framework presented in this chapter is a generic and relatively jargon-free model. It is derived from common elements of PDSA,1-3 Lean,4,5 Six Sigma,4,6,7 TeamSTEPPS,8 and the Johns Hopkins Quality and Safety Research Group,9 and further refined in a large number of successful projects10-15 and collaborative QI programs from the Society of Hospital Medicine.16-19 Whichever QI model an institution is vested in can be used to implement this framework, provided that all the elements listed here are addressed. This QI framework is also consistent with the findings from reviews and collaborative improvement experiences that outline the characteristics of interventions that are most likely to result in improved deep vein thrombosis (DVT) prophylaxis. Interventions that are active, rather than passive, appear to be the most effective, and multifaceted interventions that include an active surveillance and alert component, education, point-of-care clinical decision support, and education are more effective than single interventions used in isolation.20-25 |
Step 2: Survey Previous or Ongoing Efforts and Resources
In many ways, a multidisciplinary QI team is building, flying, and navigating an aircraft that is already airborne. It pays to know what resources are already available. Experience, precedents, and skilled individuals can significantly assist an effort. Conversely, working at odds with existing infrastructure and strategic goals can sabotage a project. Answering key questions about the landscape, available data, lessons learned, and barriers and opportunities will help the QI team identify the best approach for its improvement effort.
Review the QI Landscape
The team should make sure the elements of success are addressed and stressed in the context of the locally preferred QI framework. By surveying all the efforts already underway in VTE prevention, redundancy can be avoided and coordination ensured.
- What is the existing QI infrastructure and preferred QI framework?
- What support or services are available for this project?
- Are there any ongoing QI initiatives to learn from or leverage?
- Are there any initiatives that could bolster support for a VTE prevention effort (e.g., pursuit of Magnet Recognition Program, ventilator-associated pneumonia bundle, Surgical Care Improvement Project, TJC or NQF measures, and Partnership for Patients)?
Understand the Data
Publicly reported measures on VTE prevention and outcomes have some flaws. Despite this, alignment with these measures is desirable even as the improvement team seeks to track improved measures.
- What performance data on VTE prevention or VTE events already exist?
Identify Lessons Learned
Many VTE prevention efforts fail because of a lack of standardized guidance integrated at the point of care or due to flawed risk assessment models that either offer no guidance or are so complicated that providers bypass them. Identifying why past efforts failed to produce desired results will help guide current efforts and avoid repeating the same mistakes.
- Are there any major lessons from previous or ongoing interventions to prevent VTE?
- How successful were previous VTE risk assessments? Why? Were they integrated into order sets?
Identify Barriers and Opportunities
It is important for teams to analyze the local QI ecosystem, address the barriers pertinent to VTE prevention, and use tools that have proven effective in the past. The most effective improvement strategies focus on improving ordered prophylaxis, monitoring for care deficiencies, and intervening where deficiencies are found. Understanding the tools available for these functions can assist in devising a maximally effective new system.
- Are there ongoing VTE educational or awareness activities for medical staff?
- Are hospital policies capable of enforcing provider performance (e.g., medication reconciliation, vaccinations, VTE prophylaxis)?
- How fragmented is care in the hospital? Are intensive care units (ICUs) open or closed? Are patients geographically grouped by service or specialty?
- What are the existing practices for standardizing care transitions between settings (e.g., emergency room to floor, ICU to floor, operating room to floor, direct admissions)?
- Can precedents that have engaged patients in promoting medical staff accountability be leveraged for specific care goals?
- In what areas of the hospital are nurses engaged in promoting medical staff accountability for specific care goals (e.g., daily goals worksheet or participation in multidisciplinary rounds)?
- In what ways do clinical pharmacists participate in care delivery (e.g., participation in multidisciplinary rounds, pharmacokinetics consults, pages to providers to adjust medication dosages)?
- Could the electronic health information or paging system relay clinical information to members of the care team (e.g., alerts by email, text, page, fax, or computerized physician order entry [CPOE])?
- Is there a precedent anywhere in the institution for feeding back individual or service line performance to providers?
- Does the medical center have an electronic health record, CPOE, or digital radiology?
Step 3: Clarify Key Stakeholders and the Reporting Hierarchy
Every medical center has stakeholders who should be made aware of efforts. Often, these stakeholders are individuals, but they can also be committees, services, training programs, hospital initiatives, or departments. Typically, these groups will include:
- Pharmacy and therapeutics committees.
- Nursing groups.
- Hospitalists, hematologists, and oncologists.
- Orthopedics, surgery, and trauma leaders.
- Patient safety committee.
- Operating room and perioperative committees.
- Chief residents and residency program directors.
- Departmental committees.
Informing stakeholders of the effort and gaining their buy-in is important to boost early adoption of interventions. These stakeholders may also advance educational efforts and offer legal protection for information that is uncovered. In addition, early use of the proper reporting structures and approval processes is wise.
Step 4: Assemble an Effective Team
QI efforts often originate from a few thought leaders who see a gap between current practice and best practice. The VTE prevention team may at a minimum want to include a team leader, a team QI facilitator, process owners, information technology and health information system experts, and patient representatives.
The team leader should be a clinician the medical staff respects with some topic expertise on VTE prophylaxis and anticoagulation. While the clinician leader does not need to be a physician, having strong physician partners can bolster both the acceptance and visibility of the effort. This person is responsible for setting the agenda, a collaborative tone, and the frequency of team meetings and with communicating with administrative and medical staff committees. In addition, the team leader and the QI facilitator need to enforce constructive team dynamics.
The team leader needs commitment and contributions from other team members and a coordinated effort across the spectrum of care to move the initiative forward. The team leader and the team may need to recruit local champions based on service or hospital geography. For example, a pulmonary or critical care physician may lead efforts on VTE prophylaxis in the ICU, but a hospitalist may lead efforts on the floors or wards.
The QI facilitator, who should be someone with QI experience, plays the pivotal role of ensuring the team functions constructively and the project stays on track. This role requires project management and people management skills as well as the ability to introduce appropriate QI tools. The QI facilitator need not have mastery of QI tools at the onset of the project but should have a readiness to acquire new tools and a talent for moving projects forward. Mastery of the VTE literature is not important for this position. For smaller projects, the QI facilitator can also be the team leader. For more ambitious projects or for projects involving buy-in from disparate clinician groups, having a separate facilitator is strongly recommended.
Process owners are frontline personnel involved in providing VTE prophylaxis in the hospital and are essential for an effective team wishing to optimize VTE prevention. Ideally they represent each discipline (pharmacy, nursing, and so forth) and unit (medical, surgical, ICU, and so forth).
Information technology and health information system experts provide pivotal contributions, from performance tracking to actual QI interventions. Enlist those who can report ICD code frequencies at discharge, perform data entry, set up reports from the electronic clinical data warehouse and radiology, and serve as liaisons to health informatics. In addition, individuals skilled in run charts, statistical process control charts, and statistics are highly desirable as ad hoc members of the team.
Patient representatives provide the patient’s point of view in all aspects of the improvement effort. They can be particularly valuable when assessing adherence to mechanical prophylaxis and developing educational materials around the subset of patients who may require extended duration prophylaxis.
The key dynamic for an effective team is the removal of authority gradients. Because the perspective of every team member is potentially critical, every perspective must be heard and each team member must be comfortable expressing his or her viewpoint. Try to pick people who have reputations as collaborators.
Step 5: Define the Scope of VTE Prevention Efforts
A wide variety of patient populations are at risk for VTE. Improvement teams need to decide whether to tackle VTE prevention across the spectrum of patients at risk or limit their efforts to some special cases. For example, an improvement team could focus efforts on just critical care patients and surgical patients and let others address VTE prevention for orthopedic, general medical, oncology, and obstetrics and gynecology patients.
Focusing on a specific population has some practical advantages. The scope of the effort is more manageable, and there are fewer clinicians, order sets, and points of view to consider. In addition, teamwork may be better if efforts are limited to a certain group.
Despite these considerations, there are cases where a systemwide approach to VTE prevention may be preferred. First, patients frequently move from one setting to another or belong to more than one area of focus, leading to inconsistency and confusion. Second, focusing on only certain groups leaves large populations vulnerable. For example, if adult VTE prevention efforts focused only on surgical patients, the medical population (in whom around half of HA-VTE occurs) would not be addressed. Third, having a common institutional standard may actually make some aspects of education and implementation easier.
Step 6: Set General Goals and a Timeline
Setting a goal helps the VTE prevention team stay focused and communicate effectively with stakeholders. For clarity of purpose and to overcome initial inertia in the early stages, the team needs only to agree on general goals. Making the general goals a stretch can ensure the effort is ambitious enough to change the current process but still achievable (e.g., eliminate preventable cases of hospital-associated VTE). After seeing initial success, the team can develop a more specific and measurable aim statement for VTE prophylaxis.
The team also needs a deadline to hold itself accountable, and the timeline should be ambitious but realistic. For piloting a single improvement intervention on a single medical floor, a timeline of 12 weeks is reasonable. For spreading a series of improvement changes across an entire system, 12 to 24 months may be more appropriate.
Step 7: Use Tools and Resources To Organize Team Efforts
Appendix A contains an institutional self-assessment for VTE prevention. This tool was designed to communicate information to a consulting physician mentor in advance of a site visit, but can also be used to help improvement teams organize their efforts and their exploration of prior efforts. Appendix B also contains a sample aim statement and a variety of tools developed as part of AHRQ-funded projects to implement VTE prophylaxis.
Step 8: Use a Structured Framework for Improvement To Plan and Guide Progress
A coherent framework is as important to QI as an understanding of aeronautics is for building an airplane. There is also great value in knowing how each of the team’s activities contributes to the overall progress of the improvement effort. The framework is outlined in Figure 1.1.
- Analyze care delivery (Chapters 1 and 2). Assess past efforts and barriers to implementation. Highlight the steps in the clinical workflow where interventions will have the highest impact. Identify key barriers and failure modes and prioritize which ones to tackle as a priority. Reassess and analyze with input from local stakeholders.
- Review the evidence and assimilate general definitions for best practice from guidelines, regulatory agencies, and other sources (Chapter 3). These sources provide general standards for VTE prophylaxis in several different groups and in special populations.
- Distill the most important best practices from the guidelines and other sources and translate that information into local VTE prevention protocols and policies (Chapters 4 and 5). Protocols provide specific guidance for managing groups of patients, in an algorithmic structure that facilitates clinical decisionmaking, tailored to the local environment.21 A VTE protocol, reduced to its essence, is a standardized VTE risk assessment, linked to a menu of appropriate VTE prophylaxis options for each level of risk. Such a protocol also provides guidance for management of patients with contraindications to pharmacologic prophylaxis. This filtering or distillation of the guidelines is essential. From thousands of pages derived from a score of sources, teams must identify the most essential and important ones to reinforce in protocols.
Protocols embody the local definition of acceptable practice, and the operational definitions and details of the protocol will drive the design of order sets, measurement tools, and educational efforts. Protocol-driven order sets are ideally easy to use, provide the necessary guidance, and are positioned in such a way that the embedded guidance affects virtually all patients at key junctures (e.g., on admission, postoperatively, and on transfer from one level of care to another).20
Medical center policies add another layer of definition and reinforcement to local standards. Such policies, which will ideally state acceptable standards in more general terms, often require votes by medical center committees and medical staff to alter—and have a longer review and revision cycle. Protocols (which can take effect immediately), meanwhile, are typically more specific and easier to revise.
- Design multifaceted interventions to reinforce and integrate the protocol into practice, addressing weak links and failures in the process (Chapter 4). Robust evidence from randomized trials on interventions that improve prophylaxis and outcomes is often limited, but there is some guidance available in the literature and from previous collaborative efforts.21-25 Integrating VTE risk assessment into admission and transfer order sets is a key intervention, but this alone will not achieve the high degree of performance required to optimize prophylaxis and reduce HA-VTE. Additional interventions reinforcing the protocol, layered on top of the essential VTE prevention order sets, are the key to success. As noted above, multiple active interventions inclusive of real-time electronic or human alerts have been most successful.21-25 One example combining real-time measurement with concurrent intervention (aka measure-vention,)20 is emphasized as a reliable additional intervention to drive improved prophylaxis.
- Implement the protocol and ensure reliable delivery of best practices (Chapters 5 and 7). Implementation is where the rubber meets the road. Engaging stakeholders across the hospital, developing education plans, and using good clinical decision support, standardization, and other techniques increases the chances of optimizing prophylaxis. Ongoing evaluation, feedback, and revision and refinement are also needed.
- Track performance with metrics (Chapter 6). Set up regular data collection and charting that is reliable, inexpensive, and directly relevant to the aim. Key metrics include the prevalence of appropriate VTE prophylaxis and the incidence of HA-VTE. Measurement helps assess baseline performance, but should also inform improvement efforts longitudinally.
- Hold the gains and spread the initiative to other units, hospitals, and/or settings (Chapter 8). Holding the gains on VTE prevention and disseminating your successes is much more likely if the initial effort is done right. This includes building the right tools and infrastructure for order sets and measurement and achieving results via redesign of systems rather than by relying exclusively on interventions such as education.
Stages of the Quality Improvement Effort
Early on in the effort to improve VTE prophylaxis, the team may focus entirely on launching a well-integrated, protocol-driven VTE prevention order set. This is indeed the key foundational intervention. There are many ways to get this wrong, and only two or three ways that seem to work reliably across a variety of settings, so this guide covers how to do this in great detail.
Even the best order set, however, will fail to achieve near-perfect appropriate prophylaxis. It is important for the improvement team to recognize this in advance and get a sense of where they will be going in the future. In that spirit, this guide introduces the concept of layering multiple interventions to achieve optimal VTE prophylaxis.
The hierarchy of reliability (Table 1.1) is a construct that depicts different stages of the QI effort and the results the team can expect to have at each stage.20,27,28 While the estimates of predicted performance may vary, this hierarchy has also proven useful in glycemic control and anticoagulation improvement efforts.10,14,19
Table 1.1: Hierarchy of Reliability
| Level | Predicted Performance | |
|---|---|---|
| 1 | No protocol ("State of Nature") | 40% |
| 2 | Pseudo-protocol: decision support exists but not linked to order writing, or prompts within orders but no decision support | 50% |
| 3 | Protocol*: well integrated into orders at point of care | 65-85% |
| 4 | Enhanced protocol: complementary strategies increase use of protocol | 90% |
| 5 | Measure-vention: oversights identified and addressed in real time | 90+% |
* Protocol = standardized decision support, embedded within an order set.
The level achieved on the hierarchy of reliability is generally predictive of performance regarding the level of appropriate VTE prophylaxis. A protocol available at the point of care (Level 3), essential to embedding best practice in the clinical workflow, establishes a foundation for other interventions (Level 4) and measure-vention (Level 5).
Within the hierarchy, teams move beyond Level 1 by developing consensus on the definition of best care, embedding that definition as a protocol for standard work, and then monitoring and learning from variation from that protocol. Level 2 is achieved when protocol guidance exists but is not present at the right time or place to influence VTE prevention orders, or when there is a simple listing of options for prophylaxis in order sets with guidance about preferred options.
The first big bang for the buck in the hierarchy comes at Level 3. At this point, a best practice protocol is standardized by integrating it into the clinical workflow—most commonly by embedding it within a preprinted or electronic order set. This integration with clinical workflow gives clinicians the information they need, when and where they need it, to make an appropriate choice. The order set must earn high use by being easy to use, concise, and clear. Level 4 in the hierarchy is achieved when Level 3 is augmented by additional strategies (e.g., traditional delayed audit and feedback to care teams about protocol use) and by addressing factors that contribute to VTE (e.g., impaired mobility).
At Level 5, an improvement team uses measure-vention, a profound leap in reliability. Measure-vention represents a way to create improvement interventions directly from performance measurement. In other words, measure-vention introduces the variable of time to measurement systems: real-time measurement can highlight today's potential missed opportunities, creating an opportunity to address them immediately.20,27,28 As opposed to retrospective data collection commonly used to populate static dashboards, measure-vention techniques call for regular measurement of performance on every pertinent patient at daily or more frequent intervals. Measure-vention lends itself to automated data capture and display in run charts, especially when outliers are identified electronically.



