Appendix O. Part 2: A Clear View of Flexible Endoscope Processing: Disinfection/Sterilization Record Keeping - Implementation Guide
Slide 1: Appendix O. Part 2: A Clear View of Flexible Endoscope Processing: Disinfection/Sterilization Record Keeping

Susan Klacik B.S., CRCST, CIS, FCS
Slide 2: Objectives

After viewing this video you will be able to—
- List basic endoscope processing steps.
- Explain infection prevention guidelines for scope disinfection/sterilization, record keeping, and scope storage.
Slide 3: High-Level Disinfection/Sterilization: Instructions for Use (IFU)

- Equipment needed.
- Type of high-level disinfectant (HLD)/sterilants compatible with scope.
- Measurement of solution.
- Specific steps for thorough rinsing.
- Exact measurements for rinsing.
- Step-by step process.
Slide 4: High-Level Disinfection

- Don fresh personal protective equipment (PPE), including gloves and skin and eye protection.
- Use a closed container labeled with a biohazard symbol.
- The container should be large enough to completely immerse the endoscope in the liquid chemical sterilant (LCS)/HLD solution.
- Prepare according to the manufacturer's written IFU.
- Document:
– name of solution
– expiration date
– date of preparation
– date of use - Test the minimum recommended concentration (MRC) before each use with the disinfectant-specific test strip.
- Follow the label directions for the test strip.
- Document results.
Slide 5: Automatic Endoscope Reprocessor (AER)
- Check to ensure the scope can be processed
– Validated.
– Compatible with high-level disinfectant. - PPE used for decontamination should not be worn when handling a scope or any of the accessories after they have gone through the disinfection process.
Slide 6: Automatic Endoscope Reprocessor (AER)
- Incoming water should be filtered using bacterial retentive filters as recommended in the AER manufacturer's written IFU.
- Quality testing devices are available for many AERs to ensure the solutions are flowing.
- Testing should be performed at least weekly, after major repairs, or whenever there is a concern about equipment function.
Slide 7: Sterilization
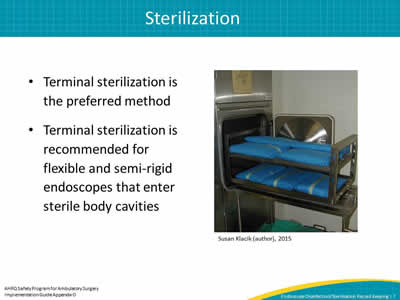
- Terminal sterilization is the preferred method.
- Terminal sterilization is recommended for flexible and semi-rigid endoscopes that enter sterile body cavities.
Susan Klacik (author), 2015
Image: showing sterilization
Slide 8: Storage IFU
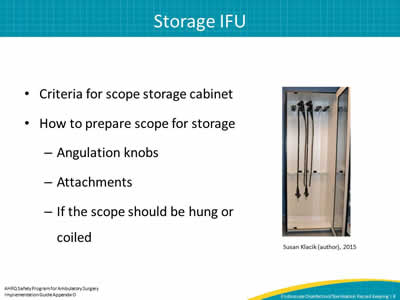
- Criteria for scope storage cabinet.
- How to prepare scope for storage
– Angulation knobs.
– Attachments.
– If the scope should be hung or coiled.
Susan Klacik (author), 2015
Image: photo of scope storage
Slide 9: Storage ST91

- Store hanging vertically with the distal tip hanging freely in a clean well-ventilated cabinet.
- Angulation lock should be in the open position.
- Leave sufficient space around scopes to prevent them hitting into one another.
- All removable parts (e.g., valves and caps) should be detached and kept with the scope.
Slide 10: Storage ST91

- A tag or label should be attached to the scope to document that the scope has been cleaned or high-level disinfected.
- The tag should be labeled with the following information:
- Date of processing.
- Name(s) of person(s) who performed the processing.
- Date of high-level disinfection.
Susan Klacik (author), 2015
Image: Tag or label shows the scope has been cleaned or disinfected
Slide 11: Storage ST91

- Channels should be dry to help prevent bacterial growth and biofilm formation.
- To help ensure that no moisture is left on or in any part of the endoscope, all channels should be flushed with 70–80 percent alcohol to facilitate drying.
- All channels should be purged with filtered medical-grade air at the correct pressure.
- Temperature and humidity in the area where the scopes are stored should be monitored.
Slide 12: Hang Time

Existing Guidelines
| Organization | Time |
|---|---|
| Association for the Advancement of Medical Instrumentation (AAMI) | Risk Assessment |
| Association for PeriOperative Registered Nurses (AORN) | 5 days |
| Canadian Standards Association | 7 days |
| Veterans Health Administration (VA) | 12 days |
Slide 13: Hang Time Risk Assessment
- Type of endoscope (with lumen or without lumen).
- Condition of the endoscope after processing (e.g., dry or wet).
- Way the endoscope was transported from processing to storage.
- Use of aseptic technique to remove the endoscope from the AER.
- Conditions of storage environment.
Slide 14: Hang Time Risk Assessment

- Excessive handling during storage.
- Manufacturer’s written IFU for storage.
- Compliance with professional organization guidelines for storage.
- Frequency of use.
- Frequency, type, and results of quality monitoring of processing.
- Quality of final rinse water (consult AAMI TIR34:2014, Water for the reprocessing of medical devices).
Slide 15: Transport of Disinfected Scopes

- Protect from recontamination.
- Don new exam gloves before removing the endoscope from the storage cabinet.
- Transport using an impervious barrier method that will prevent recontamination.
- The endoscope should be loosely coiled to prevent damage.
- The transport system should not be reused for clean transport.
Slide 16: Quality Control Ability To Track Scope Use
Identify—
- The sterilizer, AER, or soaking container.
- The date chemical sterilization or high-level disinfection was performed.
- The sterilization or high-level disinfection cycle number.
- Patient identifier.
Slide 17: Record Keeping
- Lot number.
- Specific name of the item.
- Patient identifier.
- Procedure/physician.
- LCS/HLD expiration date.
- Exposure time and temperature.
- Cycle date & time.
- Name of LCS/HLD.
- Name/initials of person performing HLD/sterilization.
- Results of quality monitoring testing.
Slide 18: References
- American National Standards Institute and Association for the Advancement of Medical Instrumentation. ANSI/AAMI ST91:2015 Flexible and semi-rigid endoscope processing in health care facilities. 2015. http://www.aami.org/productspublications/ProductDetail.aspx?ItemNumber=2477.



