Appendix P. Evaluating and Selecting Hand Hygiene Products - Implementation Guide
Slide 1: Appendix P. Evaluating and Selecting Hand Hygiene Products

Timothy Landers, Ph.D., R.N., CNP, CIC
Assistant Professor, The Ohio State University College of Nursing
Slide 2: Disclosures

Dr. Landers receives salary support and research funding from Robert Wood Johnson Foundation Nurse Faculty Scholars. He received travel support from GOJO Industries as an industry consultant.
Slide 3: Objectives

- Identify important considerations when selecting hand hygiene products.
- Apply a standardized methodology to evaluate hand hygiene products in a specific clinical setting.
Slide 4: Multimodal Strategies for Hand Hygiene
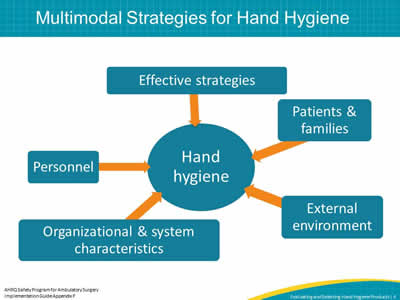
Image: multimodal strategies for hand hygiene diagram.
Slide 5: Understanding Hand Hygiene Product Evaluation

- Safety, efficacy, effectiveness.
- Regulatory framework.
- Label claims.
- Tips for evaluating claims.
Slide 6: Efficacy Versus Effectiveness
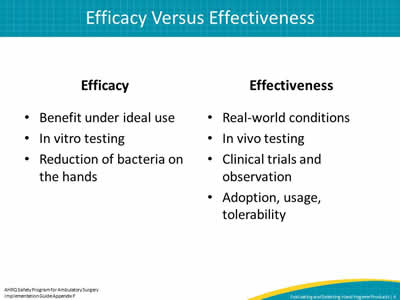
Efficacy
- Benefit under ideal use.
- In vitro testing.
- Reduction of bacteria on the hands.
Effectiveness
- Real-world conditions.
- In vivo testing.
- Clinical trials and observation.
- Adoption, usage, tolerability.
Slide 7: Regulatory Framework
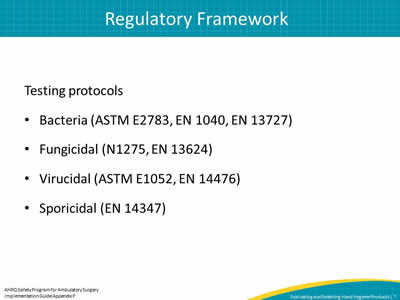
Testing protocols
- Bacteria (ASTM E2783, EN 1040, EN 13727)
- Fungicidal (N1275, EN 13624)
- Virucidal (ASTM E1052, EN 14476)
- Sporicidal (EN 14347)
Slide 8: FDA-Approved Labels

- “Handwash to help reduce bacteria that potentially can cause disease.”
- “Hand sanitizer to help reduce bacteria on the skin.”
- “Surgical hand scrub product.”
Slide 9: Evaluating Manufacturer Claims

- Inactive ingredients.
- Other components.
- Understand test methodology.
- Clinical relevance.
- Third-party evaluation.
- Dispenser technology.
- Local needs
Slide 10: Action Step: Review the Labels for Products in Current Use

- What is the labeled indication?
- What are the active and inactive ingredients?
- What data is given about in vitro testing?
Slide 11: Product Selection: Key Recommendations

- Test products locally (setting, environment, users).
- Include health care workers in product selection.
- Consider tolerability/acceptability.
Slide 12: Important Characteristics
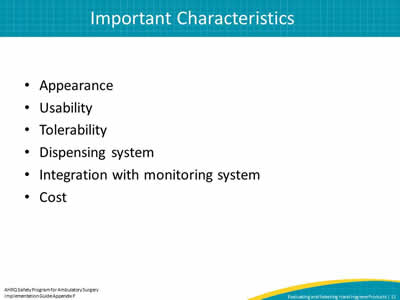
- Appearance
- Usability
- Tolerability
- Dispensing system
- Integration with monitoring system
- Cost
Slide 13: Product Evaluation World Health Organization (WHO) Method One

- Single new product evaluation.
- Completion of baseline assessment.
- Implement new product, assess at 3–5 days and 1 month.
- Full protocol specifies number users, measurement of use.
Source: World Health Organization. Tools for Evaluation and Feedback. 2009.
Slide 14: Product Evaluation WHO Method One

Part 1 – Demographics
Part 2 – Product assessment
Part 3 – Skin health assessment
Source: World Health Organization. Tools for Evaluation and Feedback. 2009.
Slide 15: Sample Questions for Local Evaluation of Hand Hygiene Products
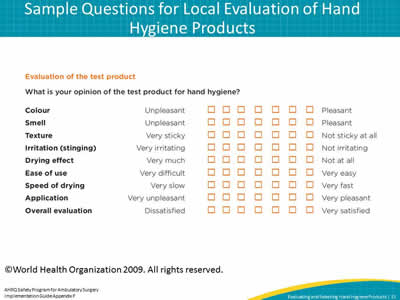
Image: WHO sample questions for evaluation of hand hygiene products
©World Health Organization 2009. All rights reserved.
Slide 16: Scales To Evaluate Skin Condition by the Observer
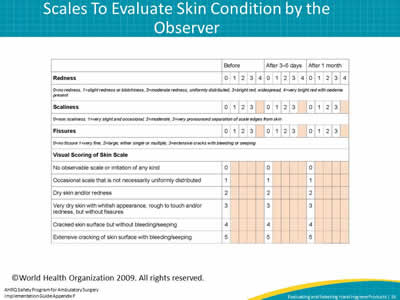
Image: This chart from the World Health Organization helps observers evaluate skin conditions at three intervals: before, after 3-5 days, and after 1 month. For redness, evaluations range from 0 for no redness to 4 for very bright read with oedema present. For scaliness, 0 is no scaliness, and 3 is very pronounced separation of scale edges from skin. For fissures, 0 is no fissure and 3 is extensive cracks with bleeding or seeping. Finally, observers evaluate the general appearance of the skin, from 0, representing no observable scale or irritation of any kind, to 5, representing extensive cracking of skin surface with bleeding/seeping.
©World Health Organization 2009. All rights reserved.
Slide 17: Product Evaluation WHO Method Two

- Two-product comparison.
- Completion of baseline assessment.
- Implement product A, assess at 3–5 days.
- Two-day “wash-out”.
- Implement product B, assess at 3–5 days.
- Full protocol specifies number of participants, measurement of use.
Source: World Health Organization. Tools for Evaluation and Feedback. 2009.
Slide 18: Product Evaluation WHO Method Two

Part 1 – Demographics
Part 2 – Product assessment
Part 3 – Skin health assessment
Source: World Health Organization. Tools for Evaluation and Feedback. 2009.
Slide 19: Action Step

Evaluate new product
- Intentionally pick participants.
- Use WHO Method One.
Evaluate current product and new product
- Use formal WHO Method 2 methodology.
Slide 20: Resources

- Association for Professionals in Infection Control and Epidemiology. Guide to Hand Hygiene Programs for Infection Prevention. 2015.
- World Health Organization. Tools for Evaluation and Feedback. 2009.



