Benefits of Subglottic Secretion Drainage Endotracheal Tubes: Slide Presentation
AHRQ Safety Program for Mechanically Ventilated Patients
Slide 1: AHRQ Safety Program for Mechanically Ventilated Patients

Benefits of Subglottic Secretion Drainage Endotracheal Tubes
Slide 2: Learning Objectives

After this session, you will be able to–
- Explain the benefits of subglottic secretion drainage endotracheal tubes (SSD-ETT).
- Access evidence and resources supporting the switch to SSD-ETT.
Slide 3: Flaws With Early Endotracheal Tubes
- Endotracheal tube/tracheostomy tube design often considered a cause of ventilator-associated pneumonia (VAP).
- Early devices with high-pressure and low-volume cuffs increased the risk of tracheal mucosal ischemia and necrosis.
- Early endotracheal tubes allowed secretions to pool below the vocal cords but above the cuff to leak into the lungs.
- While not completely eliminating the risk, tubes with barrel-shaped, low-pressure and high-volume cuffs replaced the early devices to decrease pooling of secretions.
Slide 4: SSD-ETTs1
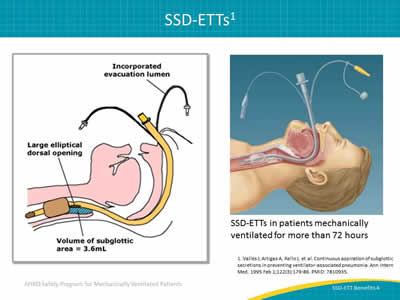
Images: Illustration identifying the key parts of a subglottic suctioning ETT. Illustration of a patient with a subglottic suctioning ETT in place.
SSD-ETTs in patients mechanically ventilated for more than 72 hours
1. Vallés J, Artigas A, Rello J, et al. Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia. Ann Intern Med 1995 Feb 1;122(3):179-86. PMID: 7810935.
Slide 5: SSD-ETT Specific VAP Prevention Guidelines

Image: Logo for SHEA, The Society for Healthcare Epidemiology of America.
Society for Healthcare Epidemiology of America2
Recommends the use of ETT with subglottic secretion drainage ports for patients likely to require more than 48 or 72 hours of intubation.
Image: Logo for Zap the VAP–Ventilator associated pneumonia.
ZAP the VAP—Ventilator Associated Pneumonia3
Subglottic secretion drainage is recommended for patients requiring mechanically ventilation for more than 72 hours.
2. Klompas M, Branson R, Eichenwald EC, et al; The Society for Healthcare Epidemiology of America. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35(8):915-36. PMID: 25026607.
3. Muscedere J, Dodek P, Keenan S, et al; VAP Guidelines Committee and the Canadian Critical Care Trials Group. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. J Crit Care 2008;23(1):126-37. PMID: 18359430.
Slide 6: SSD-ETT Specific VAP Prevention Guidelines

Image: Logo for CDC, The Centers for Disease Control and Prevention.
Centers for Disease Control and Prevention4
Recommends an ETT dorsal lumen above the endotracheal cuff to allow drainage by continuous or frequent intermittent suctioning of tracheal secretion that accumulates in patient’s subglottic area.
Image: Logo for ATS, The American Thoracic Society.
American Thoracic Society5
Recommends the use of specifically designed ETT with dorsal lumen for the continuous aspiration of subglottic secretion.
4. Tablan OC, Anderson LJ, Besser R, et al. Guidelines for preventing healthcare-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53:1-36. PMID: 15048056.
5. American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171(4):388-416. PMID: 15699079.
Slide 7: SSD-ETTs—More Recent Evidence6
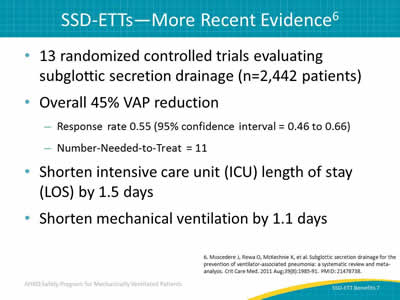
- 13 randomized controlled trials evaluating subglottic secretion drainage (n=2,442 patients).
- Overall 45% VAP reduction:
- Response rate 0.55 (95% confidence interval = 0.46 to 0.66).
- Number-Needed-to-Treat = 11.
- Shorten intensive care unit (ICU) length of stay (LOS) by 1.5 days.
- Shorten mechanical ventilation by 1.1 days.
6. Muscedere J, Rewa O, McKechnie K, et al. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med 2011 Aug;39(8):1985-91. PMID: 21478738.
Slide 8: SSD-ETTs—Cost Effectiveness Analyses
Shorr et al. states,
"Regular utilization of [closed suction system] CSS-ETs may produce significant cost savings, irrespective of the increased costs of CSS-ETs."7
Hallais et al. states,
"CSS was cost-effective even when assuming the most pessimistic scenario of VAP incidence and costs."8
7. Shorr AF, O'Malley PG. Continuous subglottic suctioning for the prevention of ventilator-associated pneumonia: potential economic implications. Chest 2001 Jan;119(1):228-35. PMID: 11157609.
8. Hallais C, Merle V, Guitard PG, et al. Is continuous subglottic suctioning cost-effective for the prevention of ventilator-associated pneumonia? Infect Control Hosp Epidemiol 2011 Feb;32(2):131-5. PMID: 21460467.
Slide 9: Subglottic Suctioning Study9

- Randomized controlled clinical trial conducted in five ICUs of the same hospital.
- The study’s purpose was to confirm the effect of subglottic secretion suctioning on VAP prevalence to assess concomitant impact on ventilator-associated conditions (VAC) and antibiotic use.
- N=352 adult patients intubated with tracheal tube allowing subglottic secretion suctioning:
- N=170, group 1 underwent suctioning.
- N=182, group 2 did not undergo suctioning.
9. Damas P, Frippiat F, Ancion A, et al. Prevention of ventilator-associated pneumonia and ventilator-associated conditions: a randomized controlled trial with subglottic secretion suctioning. Crit Care Med 2015 Jan;43(1):22-30. PMID: 25343570.
Slide 10: Results of Subglottic Suctioning Study9
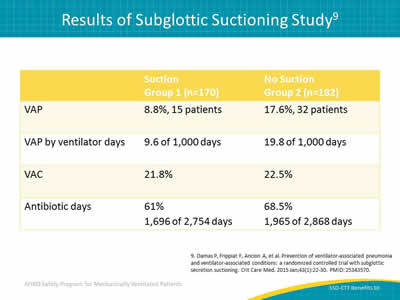
Image: Table of results of subglottic suctioning study. The patients were divided into two groups, suction and no suction. Data were gathered on four categories: VAP, VAP by ventilator days, VAC, and antibiotic days.
9. Damas P, Frippiat F, Ancion A, et al. Prevention of ventilator-associated pneumonia and ventilator-associated conditions: a randomized controlled trial with subglottic secretion suctioning. Crit Care Med 2015 Jan;43(1):22-30. PMID: 25343570.
Slide 11: Impact of Subglottic Secretion Suctioning9
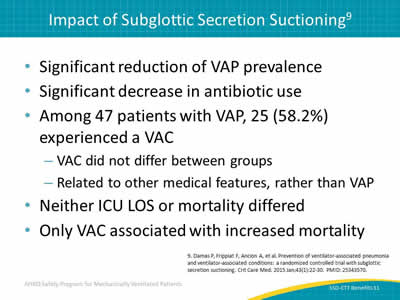
- Significant reduction of VAP prevalence.
- Significant decrease in antibiotic use.
- Among 47 patients with VAP, 25 (58.2%) experienced a VAC:
- VAC did not differ between groups.
- Related to other medical features, rather than VAP.
- Neither ICU LOS or mortality differed.
- Only VAC associated with increased mortality.
9. Damas P, Frippiat F, Ancion A, et al. Prevention of ventilator-associated pneumonia and ventilator-associated conditions: a randomized controlled trial with subglottic secretion suctioning. Crit Care Med 2015 Jan;43(1):22-30. PMID: 25343570.
Slide 12: Strategy

Identify high-risk areas of the hospital and replace standard ETTs with SSD-ETTs:
- Emergency department.
- ICU, except for brief procedures.
- Emergent intubations outside of the ICU.
- High-risk surgical procedures.
- Thoracic aortic aneurysms.
- Liver transplants.
- Anticipated postoperative open abdomen.
Slide 13: Resources—Fast Facts

- Ready to post in the unit.
- Provides quick reference to latest evidence-based protocols.
- Summarizes position of four leaders in the VAP field.
Tip: The Fast Facts for Subglottic Suctioning ETTs can be found at www.ahrq.gov/HAImvp.
Slide 14: Resources—Literature Synopsis

- Overview of current literature regarding subglottic suctioning and endotracheal tubes.
- Includes both positive and negative findings.
Tip: The Subglottic Suctioning Endotracheal Tubes Literature Synopsis can be found at www.ahrq.gov/HAImvp.
Slide 15: Questions?

Image: Picture of hanging colored tags with question marks on them.
Slide 16: References

1. Vallés J, Artigas A, Rello J, et al. Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia. Ann Intern Med 1995 Feb 1;122(3):179-86. PMID: 7810935.
2. Klompas M, Branson R, Eichenwald EC, et al; The Society for Healthcare Epidemiology of America. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35(8):915-36. PMID: 25026607.
3. Muscedere J, Dodek P, Keenan S, et al; VAP Guidelines Committee and the Canadian Critical Care Trials Group. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. J Crit Care 2008;23(1):126-37. PMID: 18359430.
Slide 17: References

4. Tablan OC, Anderson LJ, Besser R, et al. Guidelines for preventing healthcare-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53:1-36. PMID: 15048056.
5. American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171(4):388-416. PMID: 15699079.
6. Muscedere J, Rewa O, McKechnie K, et al. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med 2011 Aug;39(8):1985-91. PMID: 21478738.
Slide 18: References
7. Shorr AF, O'Malley PG. Continuous subglottic suctioning for the prevention of ventilator-associated pneumonia: potential economic implications. Chest 2001 Jan;119(1):228-35. PMID: 11157609.
8. Hallais C, Merle V, Guitard PG, et al. Is continuous subglottic suctioning cost-effective for the prevention of ventilator-associated pneumonia? Infect Control Hosp Epidemiol 2011 Feb;32(2):131-5. PMID: 21460467.
9. Damas P, Frippiat F, Ancion A, et al. Prevention of ventilator-associated pneumonia and ventilator-associated conditions: a randomized controlled trial with subglottic secretion suctioning. Crit Care Med 2015 Jan;43(1):22-30. PMID: 25343570.



